Figure 2. Iterative hepatotoxic injury perturbs CXCR7 pro-regenerative pathway in LSECs and forces the generation of a pro-fibrotic vascular niche.
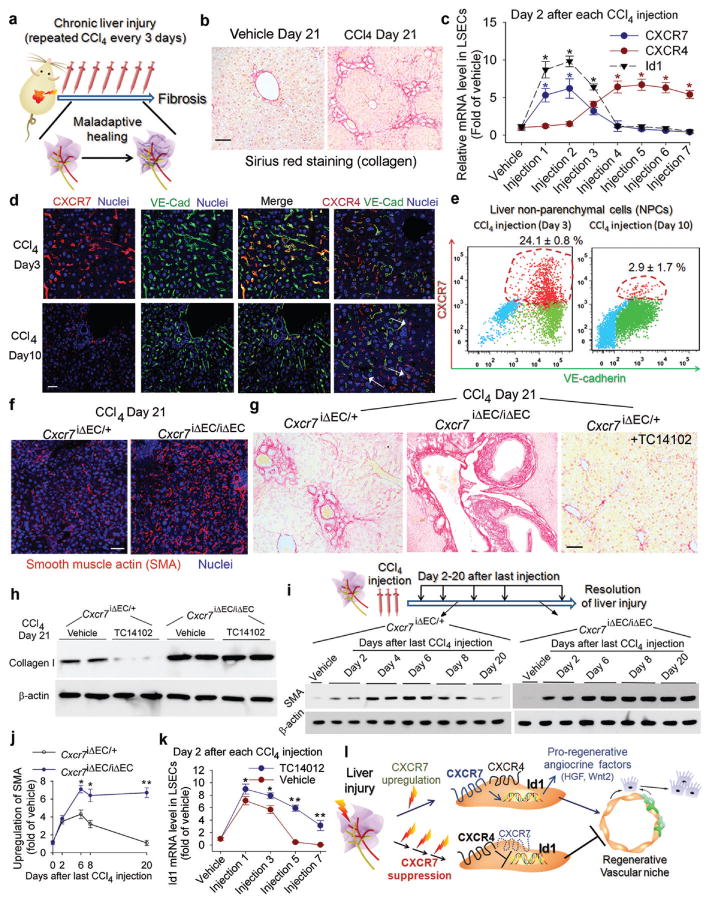
a-b) Mouse liver fibrosis is induced by repeated injection of CCl429. Sirius red staining was used to detect collagen in the injured liver. Scale br= 50 μm in Figure 2.
c-e) Chronic liver injury suppresses CXCR7 pathway and upregulates CXCR4 expression in LSECs. Quantitative PCR (c), immunostaining (d) and flow cytometry (e) showed the abrogationof CXCR7-Id1 pathway in VE-cadherin (VE-Cad)+ LSECs after chronic CCl4 injury. CXCR4 is expressed in both ECs and non-ECs (white arrow). *, P< 0.05 versus vehicle-treated mice; N=8.
f-h) CXCR7 activation in LSECs negates liver fibrosis. The extent of fibrosis was augmented in Cxcr7iΔEC/iΔEC mice, as evidenced by elevated hepatic levels of α-smooth muscle actin (SMA) and collagen I. Notably, CXCR7-selective agonist TC14012 reduced fibrosis in control but not Cxcr7iΔEC/iΔEC mice; N=7.
i, j) Impaired resolution of liver injury inCxcr7iΔEC/iΔEC mice. SMA level in the injured liver was tested to assess the resolution of injury (i). Compared to control mice, SMA level in Cxcr7iΔEC/iΔEC mice was enhanced after last CCl4 injection and remained stable afterwards. Collagen I level was similarly assessed (supplementary Figure 13); *, P < 0.05, **, P < 0.01, versus control mice; N=5.
k) CXCR7 activation restores Id1 induction in chronically injured LSECs. TC14102 prevented Id1 suppression in LSECs during repeated CCl4 injury. *, P < 0.05, **, P < 0.01, compared to vehicle group; N=7.
l) Interference with pro-regenerative CXCR7-Id1 pathway in LSECs causes pro-fibrotic transition of vascular niche. After injury, upregulation of the CXCR7-Id1 pathway in LSECs induces generation of hepatic-active angiocrine factors and stimulates regeneration. Chronic injury perturbs CXCR7-Id1 signaling, counteracting regeneration and provoking fibrogenesis.
