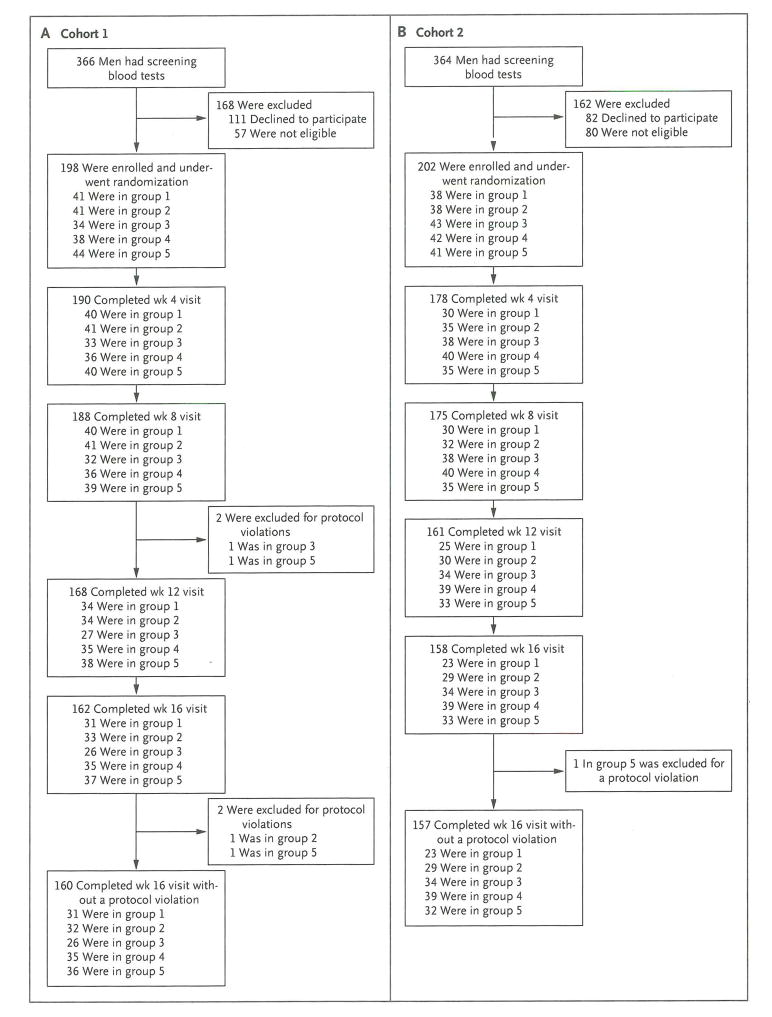
Figure 1. Recruitment of Participants and Study Completion.
Participants were recruited by sending letters to men in the local area who were identified with the use of commercially available mailing lists or by advertising in newspapers or on the Internet. A computerized program was used to randomly assign participants in permuted blocks. The block sizes were also randomly determined. Participants in cohort 1 (Panel A) were assigned to receive goserelin acetate plus placebo (group 1), 1.25 g of testosterone (group 2), 2.5 g of testosterone (group 3), 5 g of testosterone (group 4), or 10 g of testosterone (group 5) daily for 16 weeks. Participants in cohort 2 (Panel B) received the same study medications plus anastrozole at a dose of 1 mg per day. Participants who discontinued participation at week 8 or 12 were permitted to undergo repeat body-composition and strength testing that was planned for week 16. In cohort 1, eight men in group 1, five men in group 2, two men in group 3, and one man in group 4 underwent repeat body-composition and strength testing at week 8 or 12. In cohort 2, five men in group 1, two men in group 2, four men in group 3, one man in group 4, and one man in group 5 underwent repeat body-composition and strength testing at week 8 or 12.