Significance
Our findings will refine the existing concept of a simple, linear phase model of memory in terms of temporal constraints on associative memory formation. We provide evidence for an in-between situation in which a “winner-take-all” process shapes the process of stable long-term memory storage. We show that when the temporal persistence of plasticity is enabled on one pathway by virtue of the availability of proteins from another earlier or later event, potentiation of a further third pathway around the same time may trigger sufficient competition to prevent persistent potentiation on all pathways. This mimics the daily situation of hippocampal neurons, which have to cope with the learning of new events under conditions of competition that can threaten stabilization.
Abstract
Canonical models suggest that mechanisms of long-term memory consist of a synapse-specific, protein synthesis-independent induction phase (changes in synaptic weights/temporary tagging of such synapses) and, within adjacent dendritic compartments, a protein synthesis-dependent distribution phase that may accompany or immediately precede induction and whose protein products enable consolidation through synaptic capture. We now report that this distribution phase is competitive in a “winner-take-all” fashion when synapses potentiated at induction compete with each other for plasticity-related proteins. This finding highlights the importance of synaptic competition in creating stable long-lasting memory in neural networks without disruption.
Activity-dependent increases and decreases in synaptic efficacy, such as the physiological phenomena of long-term potentiation (LTP) and long-term depression, are considered to be prominent cellular mechanisms mediating learning and memory. Long-lasting persistence of synaptic strength requires protein synthesis that is achieved through transcription and translation (late LTP; L-LTP), whereas short-lasting changes do not involve protein synthesis (early LTP; E-LTP) (1). Based on this dependency on protein synthesis, a sequential model of memory has long been proposed involving the transition from E-LTP to L-LTP, and it remains an important framework for memory consolidation (2, 3). However, the newer concept of synaptic tagging and capture (STC) has changed our understanding of the necessity for a sequential framework. Instead, although memory encoding and tagging occur in real time in association with the event or stimulus to be remembered, the persistence of this trace depends also upon the capture of plasticity-related proteins (PRPs) whose synthesis can be triggered before, during, or immediately after memory encoding. The synthesis of PRPs, now thought to occur in relatively clustered dendritic domains (4, 5), creates the possibility for both synergistic (6) and competitive interactions between potential memories during the subsequent distribution phase. Synergistic effects are well-studied. However, although synaptic competition has been considered previously (7), the temporal dynamics of such competition are not well-understood. We now show that when the temporal persistence of synaptic potentiation is enabled on one pathway by virtue of the availability of PRPs from another earlier or later event, potentiation of a third pathway around the same time may trigger sufficient competition to prevent persistent potentiation on all pathways. Varying the timing of the potentiation of this third pathway enabled one or more pathways to persist whereas others do not. Thus, when the number of competing potential memories increases and the availability of PRPs is limited, a “winner-take-all” scenario appears to prevail whereby some traces persist in a stable manner whereas others do not. The use of multiple pathways models the likely situation in real life when the encoding of multiple memories can create synergistic interactions or competition for resources.
Results and Discussion
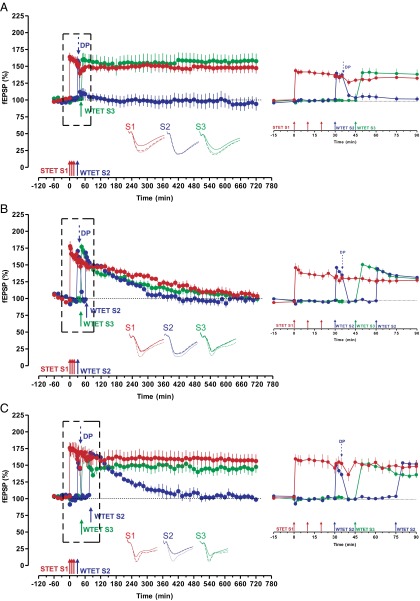
Initial interface chamber electrophysiological experiments, described in SI Methods (Fig. S1 A and B), indicated that we could induce stable LTP lasting 12 h after strong tetanization (STET) and that a weakly tetanized pathway (WTET), induced within 30 min, would then also induce L-LTP (Fig. 1A). One of the prerequisites for consistently measuring L-LTP with multiple inputs that lasts more than 10 h is the slightly high extracellular K+ concentration in the artificial cerebrospinal fluid that we used, similar to that of the original STC experiments reported by Frey and Morris (6). The stage was thus set for the key experiment, namely to induce LTP with STET, which should ordinarily result in stable L-LTP and weakly tetanize two pathways, rather than a single additional one, 30 and 45 min after STET later (Fig. 1B, blue and green filled circles). This arrangement should increase synaptic competition. The surprising result was that potentiation on all pathways declined, including the STET pathway, with potentiation decaying to baseline at 480 min. The same result was obtained when intervals of 30 and 60 min after STET were used (Fig. 1C). However, a different outcome prevailed with 30 and 75 min (Fig. 1D), with only the last tetanized pathway decaying to baseline. Synaptic competition appears, under the condition of our experiments, to occur over a period of about 60 min, but stabilization then occurs in a winner-take-all manner at the cost of memory persistence for a pathway tetanized later on (Fig. 1D, green filled circles).
Fig. 1.
Synaptic competition and its duration. (A) Time duration of late LTP (12 h) in S1 (red filled circles), followed by early LTP in S2 by WTET (blue filled circles); here, S2 expressed STC. In addition, stable control baseline potentials up to 12 h were recorded from S3 (n = 7) (for more details, see SI Methods). (B) Induction of L-LTP at 0 min in S1 (red filled circles) followed by E-LTP in S2 at 30 min (blue filled circles) and another E-LTP in S3 at 45 min (green filled circles). Here, if two weak plasticity forms (E-LTP in S2 and E-LTP in S3) compete for PRPs from a strong plasticity pathway (L-LTP), all plasticity forms decay to baseline after 4 h. This result provides strong evidence for synaptic competition in a physiological situation (n = 7). (C) The experimental design was the same as in B but E-LTP in S3 (green filled circles) was induced at 60 min (n = 7). (D) The experimental design was the same as in B and C but E-LTP in S3 was induced at 75 min (green filled circles). Interestingly, no synaptic competition was observed between S1 and S2, but S3 was not transformed to L-LTP (n = 7). (Insets) Averages of analog traces recorded from synaptic inputs S1 and S2, 30 min before (continuous line), 90 min after (dotted line), and 12 h (hatched line) after the induction of the corresponding plasticity. Error bars indicate ±SEM; fEPSP, field excitatory post synaptic potentials; n, number of experiments. (Calibration bar for all analog sweeps, 3 mV/5 ms.)
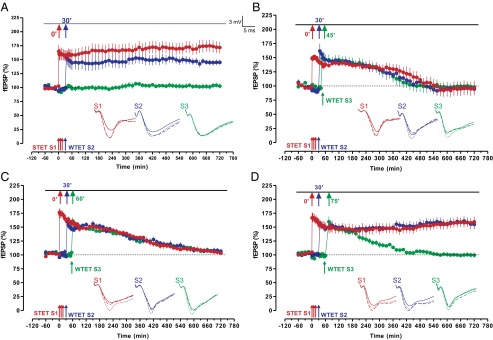
The next step was to characterize some of the determinants of synaptic competition. Competition is likely to be determined by the availability of PRPs and the number of tags that can capture them. Given that depotentiation (DP) 5 min after the induction of E-LTP can effectively reset synaptic tags (8, 9), we applied STET on distal synaptic input S1 and then, 30 min later, WTET on S2, followed 5 min later by DP stimuli (1 Hz, 250 pulses; Fig. 2A, blue filled circles). This depotentiated E-LTP should have erased a competing synaptic tag (10). Accordingly, when WTET was then applied to S3 45 min after STET, persistent potentiation was observed on both S1 and S3 (Fig. 2A)—a strikingly different result from that shown in Fig. 1B. However, if the depotentiated pathway S2 was repotentiated at 60 min after STET on S1, reinstating competition, the outcome was potentiation declining across all pathways (Fig. 2B), analogous to that shown in Fig. 1B. Moreover, reactivation of S2 at 75 min did not affect the stable potentiation seen in S1 and S3 (Fig. 2C, red and green), whereas pathway S2 itself, being repotentiated at a time point outside the synergy/competition window of the PRP distribution phase, fails to stabilize. It was intriguing to us to check whether the PRPs provided after setting the competing partners could also be involved in competition. To do this, we provided STET in S3 at 60 or 75 min after the initial WTET in S1 (Fig. 3 A and B; weak-before-strong protocol). Interestingly, the outcome was similar to that in Fig. 1 B and D. These intriguing observations provide evidence that competitive tag setting by coincidental weak activation of synapses within the temporal vicinity of strongly activated synapses triggers a graded decay of long-lasting L-LTP into a shorter form of LTP despite strong tetanic activation of that pathway. This graded and slow decline of L-LTP is a feature of the competitive maintenance mechanism. Thus, under the conditions of our experiments, the molecular boundary seems to be rather sharp and lies between 60 and 75 min poststimulus (for a statictical analyses see Tables S1–S5).
Fig. 2.
Determinants of synaptic competition. (A) Induction of L-LTP by STET at 0 min in S1 (red filled circles) followed by E-LTP by WTET in S2 at 30 min (blue filled circles). Five minutes after E-LTP in S2, low-frequency stimulation (1 Hz, 250 pulses) was applied for inducing depotentiation. Another E-LTP was induced in S3 at 45 min (green filled circles). Here, synaptic tagging between S1 and S3 was intact (n = 7). (B) The experimental design was the same as in A but the depotentiated E-LTP (filled blue circles) was again reactivated with a WTET at 60 min (n = 7). (C) The experimental design was the same as in B but the depotentiated E-LTP (filled blue circles) was reactivated at 75 min (n = 7). For clarity, relevant stimulations at a specific time period are highlighted (Left). Symbols and traces are as in Fig. 1.
Fig. 3.
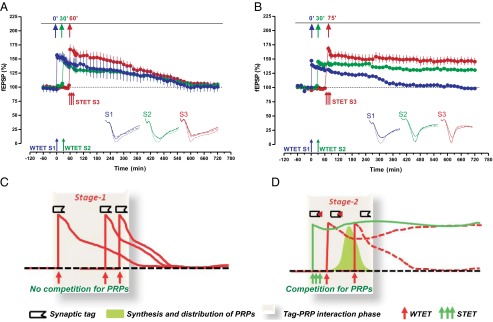
Winner-take-all phase of synaptic tagging and capture. (A) Induction of E-LTP at 0 min in S1 (blue filled circles) followed by E-LTP in S2 at 30 min (green filled circles) and L-LTP in S3 at 60 min (red filled circles). Here, if two weak plasticity forms (E-LTP in S1 and E-LTP in S2) compete for PRPs from a strong plasticity pathway (L-LTP), all plasticity forms decay to baseline after 4 h (weak before strong) (n = 5). (B) The experimental design was the same as in A but the L-LTP in S3 was induced at 75 min (filled red circles). No synaptic competition was observed between S2 (green filled circles) and S3, but S1 (blue filled circles) was not transformed to L-LTP (n = 5). (C) A key conceptual idea in STC is that two independent processes interact—tag setting and PRP up-regulation. These two processes can be triggered simultaneously or one before the other in either order. Stability occurs when the products of one process (PRP synthesis) overlap in time with the availability of the other (tag setting). Here, there is no PRP synthesis prior to or after the tag setting, leading to a no-competition phase and also to a non–memory-encoding stage. (D) The clustered synapses have a mechanism incorporated in which, due to competition, only the potentiated synapses get consolidated, and these are the ones that “survive” this competition stage, resulting in a robust form of memory.
These observations reveal a winner-take-all component of the “synergy/competition phase” of protein synthesis-dependent LTP in which the potentiated synaptic population is destined either for persistent potentiation or for a time-dependent decay. Synaptic competition in the late stage of LTP has been reported in the presence of protein synthesis inhibitors to reduce the availability of PRPs, and the suggestion has been made that such competition could provide a means for selective information storage when multiple inputs converge (7). Our results go beyond this by identifying that competition occurs even in the early phase of synaptic memory consolidation, with the outcome that either a pathway bifurcates into a stable potentiated state or it decays to baseline. This finding is in agreement with the concept that there are two contributors to the plasticity threshold essential for the effective coding of information from multiple inputs (11, 12). First, information must be encoded by means of a change in synaptic weights, and synaptic tags must be set. Second, a sufficient level of PRPs must be generated and distributed to accommodate the demand. The implication of this underlying mechanism is a model of memory in which synaptic competition serves as the means for selecting some but not all memory traces under circumstances that have the effect of limiting the disruption of those that are retained. One can think of this as a winner-take-all or “survival-of-the-fittest” principle determining what should be remembered (Fig. 3 C and D). It is in some respects analogous to lateral inhibition in the sensory systems to decrease ambiguity and to promote such perceptual processes as boundary detection.
We show here that synaptic synergy/competition can be expressed within the first hour after synaptic strengthening. Under some circumstances, competition is negligible and synergistic effects are seen with respect to the impact of PRPs upon weakly tetanized inputs. However, when multiple synapses are potentiated within a dendritic compartment such that the PRPs that can be generated and distributed are insufficient to achieve the structural changes necessary for long-term stabilization, a status of competition occurs such that either all inputs will suffer or at least a subset of these inputs will, depending on the timing of the memory induction events. The observed competition is most likely due to the increased need but reduced availability of PRPs within a specific time frame, but can be overcome by increasing the availability of PRPs over time by promoting gene activation. Indeed, activating the transcriptional machinery by metaplastic stimulations such as the priming of mGluR receptors effectively prevents synaptic competition (Fig. S2 A and B). In general, the capacity to store long-term memory in a neural network depends in part on the plasticity thresholds of “synaptic units” or “clusters,” as defined above. Although this capacity is not itself modifiable, erasing competing synaptic tags on one or more pathways can be beneficial for other potential memories created around the same time (5, 8, 12).
Methods
A total of 87 hippocampal slices prepared from 80 male Wistar rats (6- to 7-wk-old) were used for electrophysiological recordings. All procedures were approved by guidelines from the Animal Committee on Ethics in the Care and Use of Laboratory Animals of Technische Universität Braunschweig. For detailed methods and experimental procedures, see SI Methods.
Supplementary Material
Acknowledgments
We are grateful to Dr. Thomas Behnisch for helpful discussions and to Suma Gopinadhan for excellent technical support. This work was supported by the Deutsche Forschungsgemeinschaft (SA 1853/1-1; to S.S. and M.K.). S.S. was additionally supported by an Alexander von Humboldt Fellowship and National Medical Research Council Collaborative Research Grant 0041/2013. R.G.M.M. is supported by the European Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403643111/-/DCSupplemental.
References
- 1.Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44(1):5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Matthies H. In search of cellular mechanisms of memory. Prog Neurobiol. 1989;32(4):277–349. doi: 10.1016/0301-0082(89)90024-5. [DOI] [PubMed] [Google Scholar]
- 3.Matthies H. Neurobiological aspects of learning and memory. Annu Rev Psychol. 1989;40:381–404. doi: 10.1146/annurev.ps.40.020189.002121. [DOI] [PubMed] [Google Scholar]
- 4.Govindarajan A, Israely I, Huang SY, Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69(1):132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Govindarajan A, Kelleher RJ, Tonegawa S. A clustered plasticity model of long-term memory engrams. Nat Rev Neurosci. 2006;7(7):575–583. doi: 10.1038/nrn1937. [DOI] [PubMed] [Google Scholar]
- 6.Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385(6616):533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca R, Nägerl UV, Morris RG, Bonhoeffer T. Competing for memory: Hippocampal LTP under regimes of reduced protein synthesis. Neuron. 2004;44(6):1011–1020. doi: 10.1016/j.neuron.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Sajikumar S, Frey JU. Resetting of ‘synaptic tags’ is time- and activity-dependent in rat hippocampal CA1 in vitro. Neuroscience. 2004;129(2):503–507. doi: 10.1016/j.neuroscience.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Sajikumar S, Li Q, Abraham WC, Xiao ZC. Priming of short-term potentiation and synaptic tagging/capture mechanisms by ryanodine receptor activation in rat hippocampal CA1. Learn Mem. 2009;16(3):178–186. doi: 10.1101/lm.1255909. [DOI] [PubMed] [Google Scholar]
- 10.Li Q, et al. Making synapses strong: Metaplasticity prolongs associativity of long-term memory by switching synaptic tag mechanisms. Cereb Cortex. 2014;24(2):353–363. doi: 10.1093/cercor/bhs315. [DOI] [PubMed] [Google Scholar]
- 11.Sajikumar S, Korte M. Different compartments of apical CA1 dendrites have different plasticity thresholds for expressing synaptic tagging and capture. Learn Mem. 2011;18(5):327–331. doi: 10.1101/lm.2095811. [DOI] [PubMed] [Google Scholar]
- 12.Sajikumar S, Korte M. Metaplasticity governs compartmentalization of synaptic tagging and capture through brain-derived neurotrophic factor (BDNF) and protein kinase Mzeta (PKMzeta) Proc Natl Acad Sci USA. 2011;108(6):2551–2556. doi: 10.1073/pnas.1016849108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.