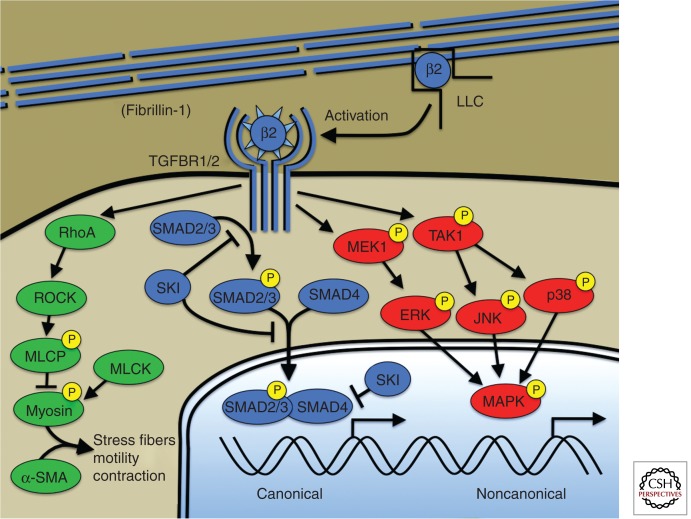
Figure 2.
Canonical and noncanonical TGF-β signaling. Microfibrils composed of fibrillin-1 bind and sequester the large latent complex (LLC) of TGF-β. Activation of TGF-β by proteases (e.g., thrombospondin-1) and/or integrin-mediated mechanisms, allows ligand binding to the TGF-β receptor. Canonical TGF-β signaling is mediated by receptor phosphorylation of R-Smads including Smad2 and Smad3. Phosphorylated R-Smads bind to the cosmad, Smad4, and translocate to the nucleus where the complex directs transcriptional events. The TGF-β repressor SKI acts at multiple levels to suppress canonical TGF-β signaling including R-Smad phosphorylation, nuclear translocation, and direct suppression of the transcriptional complex through recruitment of transcriptional corepressors. TGF-β receptors also activate so-called noncanonical signaling such as extracellular regulated kinases or ERKs. TGF-β signaling also activates RhoA activity, a known activator of smooth muscle cell contraction through myosin-actin interaction (green). Human genetic mutations have been described in all depicted members of the canonical TGF-β pathway (blue) but have not been described in noncanonical pathways required for aneurysm progression (red). Mutations causing aneurysm have been described in genes encoding smooth muscle myosin (MYH11), α-smooth muscle actin (ACTA2), and myosin light chain kinase (MYLK) but mechanistic explanation and/or the interaction between these pathways and TGF-β signaling are lacking. β2, TGF-β2; LLC, large latent complex of TGF-β; ROCK, RhoA-kinase; MLCP, myosin light chain phosphatase; MLCK, myosin light chain kinase; α-SMA, α-smooth muscle actin; ERK1, extracellular regulated protein kinase; JNK, c-Jun amino-terminal kinase; MAPK, any MAP kinase: ERK1, JNK, or p38.