Table.
Synthesis of 2-aminopyridines.
| Entry | Amine | Conditionsb | Yield 3c |
|---|---|---|---|
| a |

|
A B C |
75 78 94 |
| b |

|
A | 94 |
| c |
|
A B C |
78 77 75 |
| d |
|
A B C |
56 63 65 |
| e |
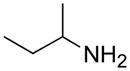
|
A B C |
65 57 79 |
| f |
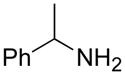
|
A B C |
75 59 61 |
| g |
|
A - |
36 48d |
| h |
|
A | 73 |
| i |
|
A | 71 |
| j |
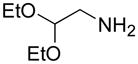
|
A | 86 |
| k |
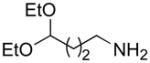
|
A | 78 |
Reactions analyzed after 48h; the spectral data for products 3a-f and 3h-j are consistent with previous reports.
A: 4.0 eq. amine, DMSO, 50 °C; B: neat amine (ca. 0.4 – 0.5M), rt; C: neat amine (ca. 0.4–0.5M), 50 °C.
percent, after chromatography.
4.0 eq. amine, DMSO, rt.
