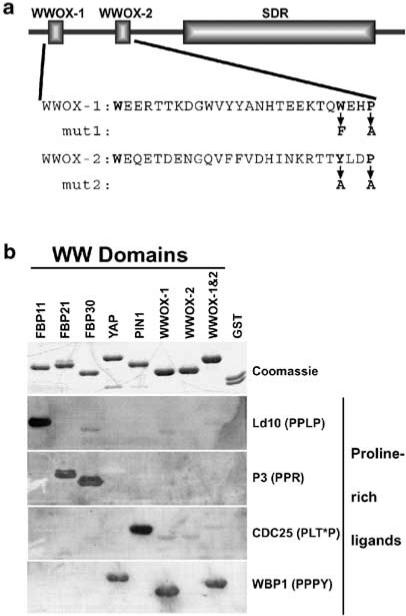
Figure 1.
WWOX WW domain I binds the PPXY ligand. (a) Full-length WWOX protein is shown schematically, highlighting the WW domains and the SDR domain. Below the schematic are the amino-acid sequences of the WW domains. Mut1 and mut2 show the amino-acid changes made in GST–WWOX-1 and -2 to inactivate the WWOX-1 and WWOX-2 domains, respectively. (b) Purified GST-fusion proteins (top panel) containing the WW domains from several different proteins were probed with each of four proline-rich ligands (bottom four panels). Equal amounts of each GST-fusion protein and GST alone were separated by SDS–PAGE and stained with Coomassie blue to visualize protein bands (Coomassie) or transferred to PVDF membrane for ligand binding. The biotinylated peptide ligands (PPLP, PPR, PLT*P, and PPPY) were bound to streptavidin-conjugated HRP prior to incubation with the membrane-bound proteins. Ligands bound by GST-fusion proteins were visualized by ECL. The complete amino-acid sequences of the peptide ligands are given in Materials and methods. T* = phosphothreonine