Abstract
Although asymptomatic left ventricular (LV) systolic dysfunction (ALVSD) is common, its phenotype and prognosis for incident heart failure (HF) and mortality are insufficiently understood. Echocardiography was done in 5,649 participants in the Cardiovascular Health Study (age 73.0 ± 5.6 years, 57.6% women). The clinical characteristics and cardiovascular risk factors of the participants with ALVSD were compared to those with normal LV function (ejection fraction ≥55%) and with symptomatic LV systolic dysfunction (SLVSD; ejection fraction <55% and a history of HF). Cox proportional hazards models were used to estimate the risk of incident HF and mortality in those with ALVSD. Also, comparisons were made among the LV ejection fraction subgroups using previously validated cutoff values (<45% and 45% to 55%), adjusting for the demographic and cardiovascular disease risk factors. Those with ALVSD (7.3%) were more likely to have cardiovascular risk factors than those in the reference group (without LV dysfunction or symptomatic HF) but less likely than those with SLVSD. The HF rate was 24 occurrences per 1,000 person-years in the reference group and 57 occurrences per 1,000 person-years in those with ALVSD. The HF rate was 45 occurrences per 1,000 person-years for those with ALVSD and mildly impaired LV dysfunction and 93 occurrences per 1,000 person-years for those with ALVSD and moderate to severe LV dysfunction. The mortality rate was 51 deaths per 1,000 person-years in the reference group, 90 deaths per 1,000 person-years in the ALVSD group, and 156 deaths per 1,000 person-years in the SLVSD group. Adjusting for covariates, compared to the reference group, ALVSD was associated with an increased risk of incident HF (hazard ratio 1.60,95% confidence interval 1.35 to 1.91), cardiovascular mortality (hazard ratio 2.13, 95% confidence interval 1.81 to 2.51), and all-cause mortality (hazard ratio 1.46, 95% confidence interval 1.29 to 1.64). In conclusion, subjects with ALVSD are characterized by a greater prevalence of cardiovascular risk factors and co-morbidities than those with normal LV function and without HF. However, the prevalence is lower than in those with SLVSD. Patients with ALVSD are at an increased risk of HF and mortality, particularly those with greater severity of LV impairment.
Heart failure (HF), with and without decreased left ventricular (LV) systolic function, is common in subjects aged ≥65 years1–4. However, asymptomatic LV systolic dysfunction (ALVSD; i.e., a decreased LV ejection fraction in the absence of HF symptoms) is an important preclinical stage of the HF continuum5. Also, ALVSD is more common than symptomatic LV systolic dysfunction (SLVSD)6–11. Evidence has suggested that ALVSD is associated with an increased risk of adverse cardiovascular outcomes, including myocardial infarction and mortality6,9,12,13. Although the clinical characteristics of SLVSD are well known, the phenotype and prognostic value of ALVSD for HF and cardiovascular mortality in older subjects have not been fully described.
In the Cardiovascular Health Study, the subjects with ALVSD had a twofold mortality risk compared to those with normal systolic function and without HF6. The purpose of the present investigation was to describe the clinical characteristics of ALVSD, to assess its effect on incident HF and cardiovascular mortality in subjects ≥65 years old, and to establish the role of prevalent coronary heart disease (CHD) and interim CHD events.
Methods
The Cardiovascular Health Study (CHS) was a prospective, community-based, observational study of subjects aged ≥65 years, identified from the Medicare enrollment lists, in 4 geographically distinct communities across the United States (Sacramento County, California; Washington County, Maryland; Forsyth County, North Carolina; and Allegheny County, Pennsylvania). The purpose of the CHS was to evaluate the cardiovascular risk factors, cardiac disease, and outcomes in free-living elderly subjects. The design and selection of subjects for the study have previously been described14. The initial cohort included 5,201 participants recruited from 1989 to 1990 and an enhanced minority cohort of 687 recruited from 1992 to 1993. The clinical information was obtained from interviews, physical examinations, and questionnaire-based assessments. The subjects also underwent evaluation of the blood biomarkers, electrocardiography, and echocardiography. The present analysis focused on 5,649 (99%) of the 5,888 participants from the initial CHS cohort with an interpretable echocardiographic assessment of LV systolic function. The participants were defined as having ALVSD if they did not have an adjudicated diagnosis of symptomatic HF at baseline and had qualitatively decreased LV systolic function (ejection fraction <55%). Those with ALVSD were compared to a reference group that included those without HF who had qualitatively normal LV systolic function (ejection fraction ≥55%) and those with SLVSD.
The diagnosis of HF was adjudicated by a review of the medical records for signs and symptoms of HF, the use of HF medications, and supporting diagnostic studies15.
Other prevalent conditions that were assessed included CHD, diabetes, hypertension, history of cerebrovascular events, ankle-arm index, and electrocardiographically determined LV hypertrophy, ST-T segment abnormalities, and atrial fibrillation. The demographic information, including age, gender, and race, was also evaluated. The electrocardiograms were analyzed as described previously16,17. The echocardiographic assessments included systolic function and valvular function and have been described in the following paragraphs. Fasting serum values were obtained for standard blood chemistry (i.e., creatinine, glucose, and lipid levels) and markers of anemia and inflammation as adjustments. The use of medications, including angiotensin-converting enzyme inhibitors, β-adrenergic blocking agents, digoxin, diuretics, calcium channel blockers, and lipid-lowering medications, was documented, and a composite of any use was included as a covariate in the multivariate models.
The echocardiographic assessment was done during the 1989 to 1990 clinic visit for the initial cohort and during the 1994 to 1995 clinic visit for the minority cohort; thus, these clinic visits were used as the baseline for the present analysis. The echocardiograms were obtained using a standardized protocol and interpreted at a core laboratory by 2 trained independent readers, who were unaware of the participants' clinical information. Qualitative assessments of the LV systolic function were categorized using previously validated cutoff values6,18 and classified as normal (ejection fraction ≥55%), mildly impaired (ejection fraction 45% to 54%), or moderate to severely impaired (ejection fraction <45%), as described previously. The inter-reader agreement of LV systolic function classification was 94%, and the intrareader agreement was 99% for 150 echocardiograms that were read twice by independent readers19.
In addition to the LV ejection fraction, LV fractional shortening18 was analyzed as a continuous measure of LV contraction and used for the confirmatory analyses.
The protocol for assessment of adverse cardiovascular outcomes during the semiannual reviews and annual examinations has been previously described20. Incident HF was confirmed if there was a physician diagnosis of HF, documentation of HF signs and symptoms, supporting diagnostic data, and medical therapy for HF. Death was confirmed by review of the medical record or death certificate or through review of the Medicare database. The Events Subcommittee adjudicated cardiovascular mortality after reviewing the medical record and death certificate.
The data are presented as the mean ± SD or percentages, as appropriate. Chi-square tests were used for categorical variables and t tests for continuous variables to compare the baseline characteristics in the participants with ALVSD, the reference group with normal LV function, and those with SLVSD. To examine which variables were associated with ALVSD independently of the other covariates (Table 1), multivariate logistic regression analyses were performed with ALVSD status as the dependent measure and the demographic, clinical, and biologic markers as predictors. Furthermore, comparisons among the participants with ALVSD were stratified by the severity of LV dysfunction (moderate to severely impaired ejection fraction [<45%] and mildly impaired ejection fraction [45% to 54%]).
Table 1. Participant characteristics.
| Characteristic | Reference (n = 4,976) |
ALVSD (n = 410) |
SLVSD (n = 104) |
p Value | |
|---|---|---|---|---|---|
|
| |||||
| ALVSD vs Reference | ALVSD vs SLVSD | ||||
| Age (years) | 72.7 ± 5.5 | 74.1 ± 6.0 | 73.9 ± 5.6 | <0.001* | 0.69 |
| Men | 1,987 (39.9%) | 278 (67.8%) | 59 (56.7%) | <0.001* | 0.034* |
| Black race | 637 (12.8%) | 46 (11.2%) | 24 (23.1%) | 0.36 | 0.002* |
| Systolic blood pressure (mm Hg) | 136.0 ± 21.3 | 136.8 ± 21.5 | 130.8 ± 21.7 | 0.42 | 0.011* |
| Diastolic blood pressure (mm Hg) | 70.7 ± 11.1 | 71.1 ± 12.6 | 66.5 ± 12.2 | 0.46 | 0.001* |
| Hypertension | 2,145 (43.2%) | 191 (46.6%) | 64 (61.5%) | 0.18 | 0.006* |
| Ankle-arm index | 1.07 ± 0.17 | 1.04 ± 0.21 | 1.00 ± 0.25 | 0.001* | 0.104 |
| Diabetes mellitus | 729 (14.8%) | 92 (22.6%) | 36 (35.6%) | <0.001* | 0.007* |
| Body mass index (kg/m2) | 26.5 ± 4.5 | 27.1 ± 4.5 | 27.1 ± 5.1 | 0.018* | 0.97 |
| Current smoker | 590 (11.9%) | 41 (10.0%) | 10 (9.6%) | 0.26 | 0.90 |
| Coronary heart disease | 767 (15.4%) | 178 (43.4%) | 77 (74.0%) | <0.001* | <0.001* |
| Left ventricular hypertrophy on electrocardiogram | 193 (4.0%) | 32 (8.3%) | 19 (20.2%) | <0.001* | 0.001* |
| ST-T abnormalities on electrocardiogram | 269 (5.6%) | 34 (8.9%) | 8 (8.3%) | 0.008* | 0.87 |
| Atrial fibrillation | 92 (1.9%) | 23 (5.6%) | 12 (11.7%) | <0.001* | 0.030* |
| Valvular abnormality | 400 (8.0%) | 48 (11.7%) | 28 (26.9%) | 0.010* | <0.001* |
| History of stroke | 178 (3.5%) | 24 (5.9%) | 14 (13.5%) | 0.017* | 0.008* |
| Fractional shortening (%) | 42.6 ± 7.7 | 33.4 ± 9.3 | 25.7 ± 10.0 | <0.001* | <0.001* |
| Angiotensin-converting enzyme inhibitor | 289 (5.8%) | 38 (9.3%) | 40 (38.5%) | 0.004* | <0.001* |
| β Blocker | 626 (12.6%) | 64 (15.7%) | 9 (8.7%) | 0.072 | 0.067 |
| Diuretic | 1,199 (24.1%) | 116 (28.4%) | 82 (78.9%) | 0.051 | <0.001* |
| Antihypertensive | 2,198 (44.2%) | 230 (56.4%) | 97 (93.3%) | <0.001* | <0.001* |
| Digoxin | 301 (6.1%) | 56 (13.7%) | 53 (51.0%) | <0.001* | <0.001* |
| Lipid-lowering drugs | 269 (5.4%) | 25 (6.1%) | 2 (1.9%) | 0.54 | 0.087 |
| Creatinine (mg/dl) | 1.04 ± 0.32 | 1.20 ± 0.55 | 1.41 ± 0.91 | <0.001* | 0.003* |
| Cholesterol (mg/dl) | 212.6 ± 38.8 | 205.5 ± 40.7 | 199.5 ± 42.4 | <0.001* | 0.20 |
| Glucose (mg/dl) | 109.6 ± 34.6 | 117.4 ± 44.2 | 121.2 ± 43.3 | <0.001* | 0.45 |
| Hemoglobin (g/dl) | 14.0 ± 1.3 | 14.4 ± 1.5 | 13.7 ± 1.7 | <0.001* | <0.001* |
| C-reactive protein (mg/L) | 4.46 ± 7.79 | 5.66 ± 9.82 | 9.14 ± 11.11 | 0.003* | 0.002* |
| Interleukin-6 (pg/ml) | 2.12 ± 1.85 | 2.51 ± 2.06 | 3.20 ± 2.17 | <0.001* | 0.005* |
| Fibrinogen (mg/dl) | 320.5 ± 65.3 | 328.1 ± 70.1 | 352.5 ± 80.6 | 0.026* | 0.003* |
Statistically significant.
The incidence of HF, cardiovascular death, and all-cause mortality are reported as percentages. Cox proportional hazards models were used to determine the hazard ratio (HR) and 95% confidence intervals (CIs) as a measure of the relative risk of incident events. The models were then adjusted for the demographic variables, including age, gender, and race. Subsequently, the covariates that have been previously validated as predictors of incident HF in the CHS were added to the models as adjustments,21 including CHD, valvular abnormality, a history of a cerebrovascular event, diabetes, systolic blood pressure, ankle-arm index, glucose, creatinine, C-reactive protein, electrocardiographically determined LV hypertrophy, ST-T segment abnormalities, atrial fibrillation, and the composite medication variable. Because the participants might have begun taking medications during the course of follow-up, we also adjusted for the composite medication variable that was updated over time if they had ever used the medications included in the composite variable. To account for the effect of valvular disease, medication status, and the presence of CHD at study entry, separate models were used, stratifying for these measures.
The models were constructed to analyze the effect of interim cardiovascular events on the subsequent outcomes. We examined the role of interim events as a covariate in the time-dependent Cox proportional hazards models. Two-tailed probabilities were examined at a 2-sided α level of <0.05. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, Chicago, Illinois) and STATA (StataCorp, College Station, Texas).
Results
At baseline, ALVSD was present in 410 participants (7.3%), of whom 65.6% had mildly impaired LV function and 34.4% had moderate to severely impaired LV function.
Two comparison groups were used, a “reference group” with normal LV function and no history of HF (n = 4,976), and a second group with SLVSD (n = 104). HF with a preserved ejection fraction (≥55%) was present in 159 subjects (2.8%), and these patients were not included in the remainder of the analyses.
The baseline characteristics for those with ALVSD are listed in Table 1. Compared to the reference group, ALVSD was associated with older age, male gender, and multiple subclinical and clinical disease characteristics. Multivariate analyses indicated that the following variables were associated with ALVSD after adjusting for other demographic, clinical, and biologic markers: male gender (odds ratio [OR] 2.56, 95% CI 1.82 to 3.60), CHD (OR 3.93, 95% CI 3.00 to 5.13), LV hypertrophy (OR 1.96, 95% CI 1.24 to 3.10), atrial fibrillation (OR 2.00, 95% CI 1.03 to 3.90), BMI (OR 1.05, 95% CI 1.02 to 1.09), and angiotensin-converting enzyme inhibitor use (OR 1.64, 95% CI 1.04 to 2.58).
Compared to the participants with SLVSD, ALVSD was associated with male gender, nonblack race, and greater systolic and diastolic blood pressure but fewer characteristics of subclinical and clinical disease (Table 1). Of those with SLVSD, 38 (36.5%) had mildly impaired and 66 (63.5%) had moderate to severely impaired LV systolic function, the approximate inverse of the severity of LV systolic function noted for those with ALVSD. Multivariate analyses indicated that the following variables were associated with ALVSD, compared to SLVSD, after adjusting for the other demographic, clinical, and biologic markers: less CHD (OR 0.27, 95% CI 0.13 to 0.60), systolic blood pressure (OR 1.03, 95% CI 1.00 to 1.05), less angiotensin-converting enzyme inhibitor use (OR 0.40, 95% CI 0.16 to 0.96), less diuretic use (OR 0.18, 95% CI 0.07 to 0.46), and less digoxin use (OR 0.33, 95% CI 0.14 to 0.80).
The characteristics of the participants with ALVSD stratified by the severity of LV dysfunction are listed in Table 2. ALVSD with moderate to severe LV dysfunction (LV ejection fraction <45%) was associated with a lower ankle-arm index, less hypertension, less current smoking, CHD, LV hypertrophy, and the use of digoxin compared to those with mild LV dysfunction (LV ejection fraction 45% to 55%). Multivariate analyses revealed that, after adjusting for other demographic, clinical, and biologic markers, male gender (OR 2.31, 95% CI 1.04 to 5.15), CHD (OR 2.80, 95% CI 1.58 to 4.94), current nonsmoker (OR 0.28, 95% CI 0.10 to 0.78), and lower hemoglobin (OR 0.81, 95% CI 0.66 to 0.99) were associated with moderate to severe LV dysfunction compared to mild LV dysfunction among the participants with ALVSD.
Table 2. Comparison of baseline characteristics stratified by left ventricular (LV) dysfunction.
| Characteristic | Mildly Impaired (n = 269) |
Moderate/Severely Impaired (n = 141) |
p Value |
|---|---|---|---|
| Age (years) | 73.9 ± 6.1 | 74.7 ± 5.9 | 0.20 |
| Men | 174 (64.7%) | 104 (73.8%) | 0.062 |
| Black race | 31 (11.5%) | 15 (10.6%) | 0.79 |
| Systolic blood pressure (mm Hg) | 136.1 ± 20.7 | 138.3 ± 23.0 | 0.31 |
| Diastolic blood pressure (mm Hg) | 70.4 ± 12.6 | 72.5 ± 12.5 | 0.10 |
| Ankle-arm index | 1.06 ± 0.20 | 1.00 ± 0.22 | 0.003* |
| Hypertension | 136 (50.6%) | 55 (39.0%) | 0.026* |
| Diabetes mellitus | 63 (23.5%) | 29 (20.7%) | 0.52 |
| Body mass index (kg/m2) | 27.2 ± 4.6 | 26.8 ± 4.4 | 0.35 |
| Current smoker | 33 (12.3%) | 8 (5.7%) | 0.034* |
| Coronary heart disease | 97 (36.1%) | 81 (57.5%) | <0.001* |
| Left ventricular hypertrophy on electrocardiogram | 16 (6.3%) | 16 (12.3%) | 0.044* |
| ST-T abnormalities on electrocardiogram | 22 (8.7%) | 12 (9.2%) | 0.85 |
| Atrial fibrillation | 13 (4.8%) | 10 (7.1%) | 0.35 |
| Valvular abnormality | 27 (10.0%) | 21 (14.9%) | 0.15 |
| History of stroke | 14 (5.2%) | 10 (7.1%) | |
| Fractional shortening (%) | 35.5 ± 8.05 | 29.4 ± 10.2 | |
| Angiotensin-converting enzyme inhibitor | 25 (9.4%) | 13 (9.2%) | 0.96 |
| β Blocker | 45 (16.9%) | 19 (13.5%) | 0.37 |
| Diuretic | 74 (27.7%) | 42 (29.8%) | 0.66 |
| Antihypertensive | 154 (57.7%) | 76 (53.9%) | 0.46 |
| Digoxin | 28 (10.5%) | 28 (19.9%) | 0.009* |
| Lipid-lowering medication | 14 (5.2%) | 11 (7.8%) | 0.31 |
| Creatinine (mg/dl) | 1.20 ± 0.63 | 1.21 ± 0.37 | 0.78 |
| Cholesterol (mg/dl) | 206.9 ± 40.0 | 202.6 ± 42.03 | 0.31 |
| Glucose (mg/dl) | 120.0 ± 50.9 | 112.4 ± 26.6 | 0.10 |
| Hemoglobin (g/dl) | 14.38 ± 1.51 | 14.45 ± 1.38 | 0.63 |
| C-reactive protein (mg/L) | 5.42 ± 9.20 | 6.12 ± 10.93 | 0.50 |
| Interleukin-6 (pg/ml) | 2.46 ± 2.13 | 2.60 ± 1.91 | 0.53 |
| Fibrinogen (mg/dl) | 323.3 ± 65.5 | 337.5 ± 77.8 | 0.053 |
Mildly impaired correlated with LV ejection fraction of 45-54%; moderate/severely impaired correlated with LV ejection fraction of <45%.
Statistically significant.
A total of 1559 cases of incident HF developed during a median follow-up of 11.7 years. Incident HF occurred more often in the participants with ALVSD than in the reference group (unadjusted HR 2.52, 95% CI 2.16 to 2.93). The HF rate was 57 occurrences per 1,000 person-years in those with ALVSD compared to 24 per 1,000 person-years in the reference group. The incidence of HF in those with moderately to severely impaired LV function (93 occurrences per 1,000 person years) was more than double that of those with mildly impaired LV function (45 occurrences per 1,000 person-years). Multivariate analyses adjusting for the demographic variables (i.e., age, gender, and race) revealed that ALVSD was a significant predictor of incident HF (HR 2.17, 95% CI 1.85 to 2.53). In the fully adjusted models that accounted for cardiovascular risk factors, creatinine, inflammation, CHD status, history of cerebrovascular accident, LV hypertrophy, atrial fibrillation, ALVSD remained significantly predictive of incident HF (HR 1.60; 95% CI 1.35 to 1.91). As listed in Table 3 and shown in Figure 1, the risk was significant even for those with ALVSD and mild LV dysfunction (HR 1.31, 95% CI 1.06 to 1.63, p = 0.014), although less than that with moderate to severe LV dysfunction (HR 2.34, 95% CI 1.81 to 3.02, p <0.001).
Table 3. Association between asymptomatic left ventricular systolic dysfunction (ASLVD) and adverse clinical outcomes.
| Variable | RR (95% CI) | |
|---|---|---|
|
| ||
| Mildly Impaired (n = 269) |
Moderate/Severely Impaired (n = 141) |
|
| Incident heart failure | ||
| Unadjusted | 1.94 (1.60–2.36) | 4.30 (3.42–5.42) |
| Fully adjusted | 1.31 (1.06–1.63) | 2.34 (1.81–3.02) |
| Cardiovascular mortality | ||
| Unadjusted | 2.60 (2.16–3.12) | 4.48 (3.57–5.63) |
| Fully adjusted | 1.94 (1.59–2.37) | 2.53 (1.98–3.24) |
| All-cause mortality | ||
| Unadjusted | 1.67 (1.46–1.91) | 2.75 (2.31–3.27) |
| Fully adjusted | 1.33 (1.15–1.54) | 1.76 (1.46–2.13) |
Mildly impaired correlated with LV ejection fraction of 45–54%; moderate/severely impaired correlated with LV ejection fraction of <45%
RR = relative risk.
Figure 1.
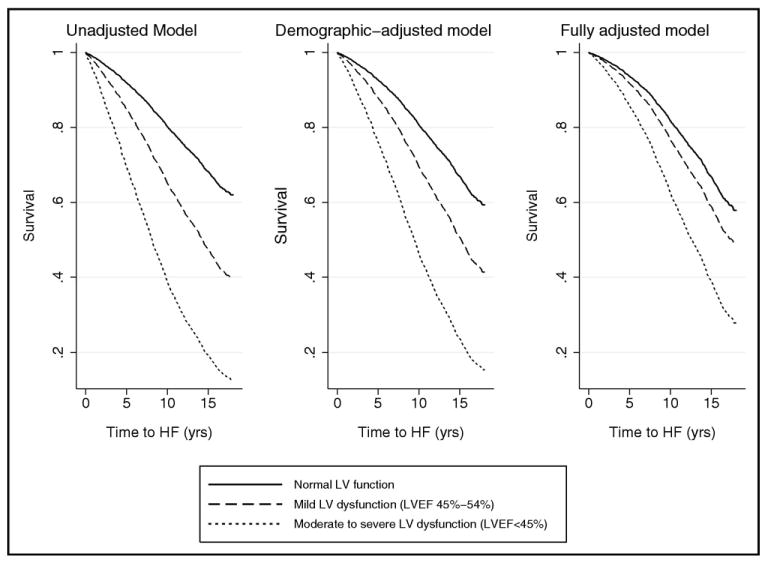
Survival curves displaying longitudinal association of ASLVD with incident HF. Participants with normal LV ejection fraction compared to those with mild and moderate to severely impaired LV dysfunction. Multivariate Cox proportional hazards models were used, adjusting for demographics (Left) and cardiovascular disease status and risk factors (Right).
Sensitivity analyses were conducted by excluding those patients with valvular disease, and similar risks associated with ALVSD for incident HF were found (adjusted HR 1.48, 95% CI 1.23 to 1.79). Excluding participants using HF medications (i.e., angiotensin-converting enzyme inhibitors, β-blocking agents, diuretics, digoxin, calcium channel blockers, and lipid-lowering medications) altered the covariate-adjusted risk estimates for incident HF (adjusted HR 1.92, 95% CI 1.44 to 2.56). When stratifying the group by CHD status, ALVSD was predictive of incident HF in patients free of CHD at screening (adjusted HR 1.88, 95% CI 1.50 to 2.36). The covariate-adjusted risk of incident HF was less strong among patients with CHD at screening (adjusted HR 1.33, 95% CI 1.03 to 1.72), which was confirmed by a significant interaction term between CHD status and ALVSD for incident HF (p interaction = 0.046).
To validate these findings based on a qualitative assessment of LV dysfunction, we examined the predictive value of LV fractional shortening (a continuous quantitative measure of systolic function) for incident HF. The lowest quintile of fractional shortening (<35.3%) was associated with an increased risk of HF compared to the upper quintile (HR 1.69, 95% CI 1.40 to 2.03) and remained significant when adjusting for covariates (HR 1.53, 95% CI 1.25 to 1.88 compared to the upper quintile; >48.8%; p <0.001).
A total of 3,596 participants died during follow-up (3,498 excluding those with SLVSD). The all-cause mortality rate (Figure 2) was 90 deaths per 1,000 person-years for the participants with ALVSD compared to 51 deaths per 1,000 person-years in the reference group. In contrast, 156 deaths per 1,000 person-years occurred in the participants with SLVSD. When stratified by the severity of LV dysfunction, the rate was 78 deaths per 1,000 person-years for participants with ALVSD and mildly impaired LV dysfunction and 118 deaths per 1,000 person-years for those with ALVSD and moderate to severe LV dysfunction.
Figure 2.
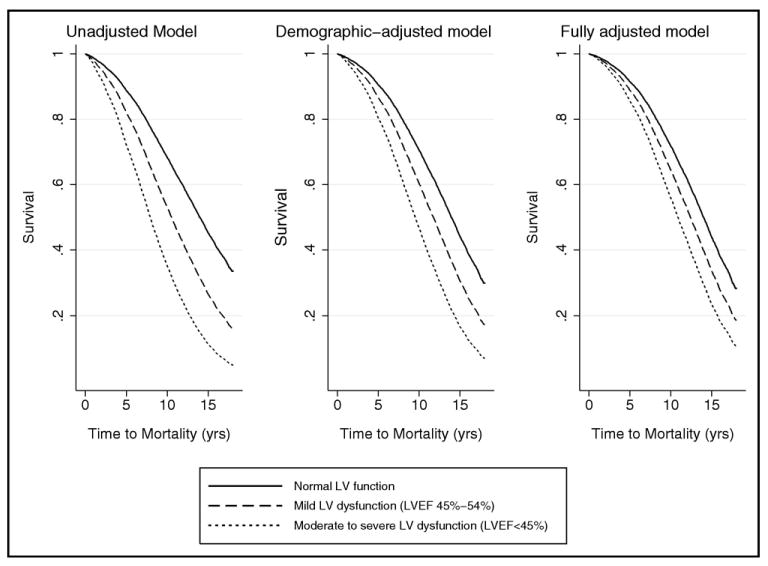
Survival curves displaying longitudinal association of ASLVD with all-cause mortality. Participants with normal LV ejection fraction compared to those with mild and moderate to severely impaired LV dysfunction. Multivariate Cox proportional hazards models used, adjusting for demographics (Left) and cardiovascular disease status and risk factors (Right).
The association of ALVSD with all-cause mortality risk (unadjusted HR 1.96, 95% CI 1.75 to 2.19) remained significant after adjustment for covariates (demographic-adjusted HR 1.66, 95% CI 1.49 to 1.86; fully adjusted HR 1.46, 95% CI 1.29 to 1.64). Elevated mortality risks were observed in those with ALVSD and mild LV dysfunction and those with ALVSD and moderate to severe LV dysfunction (Table 3). The fully adjusted relative risk of all-cause mortality remained significant for both levels of LV dysfunction (Table 3).
When comparing ALVSD and SLVSD, all-cause mortality was significantly lower in the ALVSD group than in the SLVSD group (unadjusted HR 0.50, 95% CI 0.40 to 0.63; adjusted HR 0.66, 95% CI 0.49 to 0.87).
The association of ALVSD with all-cause mortality remained unchanged when those with moderate to severe aortic or mitral stenosis or regurgitation were excluded from the analysis or when patients were stratified by CHD status at baseline. The exclusion of participants taking HF medications somewhat altered the association (HR 1.63, 95% CI 1.36 to 1.97).
Cardiovascular mortality occurred in 1,409 participants during follow-up (1,343 excluding those with SLVSD). The cardiovascular mortality rate was 18 deaths per 1,000 person-years in the reference group and 52 deaths per 1,000 person-years for participants with ALVSD. In contrast, the rate was 105 deaths per 1,000 person-years for participants with SLVSD. When stratified by severity of LV dysfunction, the rate was 44 deaths per 1,000 person-years for participants with ALVSD with mildly impaired LV dysfunction and 70 deaths per 1,000 person-years for participants with ALVSD with moderate to severe LV dysfunction.
The association of ALVSD with an increased risk of cardiovascular mortality remained significant after adjustment for demographics (HR 2.61, 95% CI 2.24 to 3.03) and in the fully adjusted model (HR 2.13, 95% CI 1.81 to 2.51; Figure 3). The participants with ALVSD and moderate to severe LV dysfunction and those with mildly impaired LV dysfunction had an increased risk of cardiovascular mortality in the fully adjusted models (Table 3). Comparing ALVSD with SLVSD, cardiovascular mortality was significantly lower in the ALVSD group than in the SLVSD group (unadjusted HR 0.43, 95% CI 0.33 to 0.57, adjusted HR 0.71, 95% CI 0.50 to 1.01), similar to the results for all-cause mortality.
Figure 3.
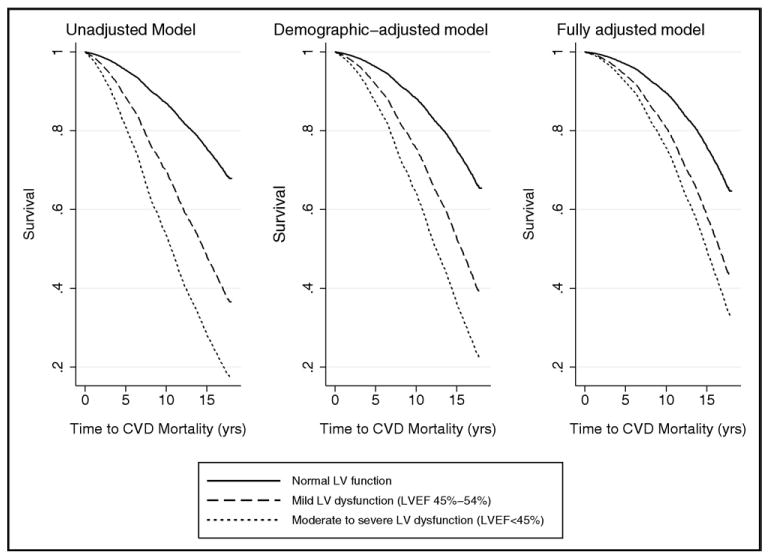
Survival curves displaying longitudinal association of ASLVD with cardiovascular disease-related mortality. Participants with normal LV ejection fraction compared to those with mild and moderate to severely impaired LV dysfunction. Multivariate Cox proportional hazards models used, adjusting for demographics (Left) and cardiovascular disease status and risk factors (Right).
The associations of ALVSD with cardiovascular mortality remained essentially unchanged when those with moderate to severe aortic or mitral stenosis or regurgitation were excluded from the analysis or when the patients were stratified by CHD status at baseline. The exclusion of participants taking HF medications somewhat changed the association (HR 2.20, 95% CI 1.67 to 2.90).
We tested whether interim myocardial infarction played a role in the association between ALVSD and incident HF. Among the participants with ALVSD, 115 patients (28.1%) had a history of myocardial infarction at study entry, and 79 (19.3%) had a myocardial infarction during follow-up. Only 12 of these 79 myocardial infarction events had preceded the onset of HF; 24 patients experienced simultaneous myocardial infarction and incident HF, 17 developed myocardial infarction after incident HF, and 26 had a new-onset myocardial infarction but no subsequent HF. Among the participants in the reference group, 320 (6.4%) had a history of myocardial infarction at study entry and 766 (15.4%) had myocardial infarction during follow-up. A total of 107 of these myocardial infarction events preceded the onset of incident HF, 230 coincided with incident HF, and 110 followed incident HF; 319 patients had a new-onset myocardial infarction without subsequent HF.
When myocardial infarction was included as a time-varying covariate, ALVSD remained associated with incident HF (unadjusted HR 1.61, 95% CI 1.38 to 1.89; fully adjusted HR 1.44, 95% CI 1.21 to 1.71), all-cause mortality (unadjusted HR 1.60, 95% CI 1.43 to 1.79; fully adjusted HR 1.39, 95% CI 1.23 to 1.57), and cardiovascular mortality (unadjusted HR 2.09, 95% CI 1.80 to 2.44; fully adjusted HR 1.92, 95% CI 1.63 to 2.27). The results replacing CHD as the time-varying covariate were similar.
Discussion
In the present study, we have reported the prevalence and phenotypic characteristics of ALVSD in elderly community-based subjects. Furthermore, we have demonstrated that ALVSD is an independent risk factor for the development of incident HF, mortality from cardiovascular causes, and all-cause mortality. We also demonstrated that the severity of LV dysfunction in those with ALVSD is related to the risk of incident HF and mortality.
ALVSD was observed in 7.3% of this elderly population, 4 times as prevalent as SLVSD (1.8%) and 3 times as prevalent as HF with preserved ejection fraction (2.8%). Compared to previous epidemiologic studies, we found a greater prevalence of those with ALVSD6–11. This might reflect the elderly population studied, as well as the inclusion of mild impairment of LV systolic function in our definition of ALVSD. ALVSD with mild LV dysfunction was more common than ALVSD with moderate to severely impaired LV dysfunction. This might reflect a continuum of disease progression that shows a trend toward a greater prevalence of symptomatic HF at baseline in those with worse LV function. Alternatively, survivor effects might have decreased the proportion of those with severe LV dysfunction in this elderly population cohort.
Although myocardial infarction at study entry was more common (28%) in participants with ALVSD than in the reference population (6%), the proportions of those with myocardial infarction during follow-up (15% reference group, 19% ALVSD) and those with new myocardial infarction accompanying new-onset HF (30% in both reference and ALSVD groups) were essentially identical, as was the proportion of those with myocardial infarction remotely preceding HF (14% in reference group and 15% in ALVSD group). Moreover, our analyses of the data established that the association of ALVSD with new HF is independent of myocardial infarction and that the association of interim events (including myocardial infarction) with all-cause or cardiovascular mortality and with incident myocardial infarction did not differ between the reference groups and participants with ALVSD.
The results of our study have established that ALVSD is a distinct category along the HF spectrum with phenotypic characteristics that are intermediate between those with normal LV systolic function and without HF and those with SLVSD, because those with ALVSD were more likely to have cardiovascular risk factors than those in the reference group but less likely than those with SLVSD. They were also more likely to have mild LVSD than moderate or severe LVSD compared to the SLVSD group, for which moderate or severe LVSD was more common.
Our findings support the American College of Cardiology and American Heart Association classification of ALVSD as a preclinical stage of the HF spectrum5. These results also complement those of the Rochester Epidemiology Project, which found that participants with prevalent cardiovascular risk factors and co-morbidities were more likely to have LV systolic dysfunction9.
In this elderly population, the risk of HF and mortality associated with ALVSD was stronger in those with moderate to severe LV dysfunction than mild LV dysfunction. These findings are consistent with observations from the Framingham Heart Study, in which both mild LV dysfunction and moderate to severe LV dysfunction displayed elevated risk of incident HF13. Participants with ALVSD had worse mortality and cardiovascular mortality compared to the reference group with normal LV function. The results of the present study concur with the findings from Hobbs et al,12 who noted that those with ALVSD had worse outcomes than those without LV dysfunction or HF. Our findings were also consistent with and provide long-term follow-up of trends noted in earlier CHS analyses by Gottdiener et al6. The present study has also demonstrated that ALVSD is predictive of incident HF and mortality, independent of interim myocardial infarction events or CHD.
In addition to extending the findings of some other population-based studies, the principal strengths of the Cardiovascular Health Study lie in the wide geographic representation, racial diversity, and extensive follow-up of subjects. Furthermore, the CHS participants underwent extensive entry examinations, and the study participants were closely followed for the development of well-characterized clinical end points. The analysis of risk in our study was adjusted for a more comprehensive set of covariates than in other studies12,13. In addition, the present study reported an analysis of the effect of interim events on the subsequent outcomes. Compared to the population in the Studies of Left Ventricular Dysfunction (SOLVD)-Prevention trial, those enrolled in the CHS represented community-based free-living elderly and had no history of clinically diagnosed HF at enrollment, and 33% of patients in SOLVD-Prevention group were New York Heart Association functional class II, and the patients were selected according to their willingness to participate in a clinical trial22.
The present study was limited in the adjudication of HF, because we relied on the physician diagnoses ascertained at follow-up visits and review of the participant's medical record. It is possible that this might lead to an underestimation or overestimation of events. Moreover, ascertainment of cardiovascular mortality from medical records and death certificates might be subject to error. Another potential limitation of the present study was that the evaluation of LV systolic function was categorized according to the qualitative and categorical visual assessments rather than quantitative calculation of ejection fraction. However, robust associations were found between LV systolic function and outcome, as well as other phenotypic characteristics. Moreover, we noted similar results when LV fractional shortening, a continuous measure of LV contraction, was used to evaluate the outcome measures. Furthermore, just as is the case with visual qualitative assessment, the quantitative calculation of ejection fraction using echocardiography remains subjectively dependent on reader identification of endocardial surfaces and selection of anatomically appropriate image planes. Moreover, quantitative algorithms (e.g., method of disks using apical window biplane images) actually use less image data than qualitative global assessment of all available image planes.
Acknowledgments
This study was supported by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant U01 HL080295, and grant R0-1 HL079376 from the National Heart, Lung, and Blood Institute (Bethesda, Maryland), with an additional contribution from the National Institute of Neurological Disorders and Stroke, Bethesda, Maryland.
Footnotes
A full list of the principal Cardiovascular Health Study Investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
References
- 1.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 2.Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc. 1997;45:968–974. doi: 10.1111/j.1532-5415.1997.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 3.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 4.Thomas S, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Heart Fail Clin. 2007;3:381–387. doi: 10.1016/j.hfc.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt SA. ACC/AHA guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–e82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function: the Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 7.Mosterd A, Cost B, Hoes AW, de Bruijne MC, Deckers JW, Hofman A, Grobbee DE. Prevalence of heart failure and left ventricular dysfunction in the general population: the Rotterdam study. Eur Heart J. 1999;20:447–455. [PubMed] [Google Scholar]
- 8.Nielsen OW, Hilden J, Larsen CT, Hansen JF. Cross sectional study estimating prevalence of heart failure and left ventricular systolic dysfunction in community patients at risk. Heart. 2001;86:172–178. doi: 10.1136/heart.86.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 10.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33:1948–1955. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 11.Wang TJ, Levy D, Benjamin EJ, Vasan RS. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138:907–916. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs FD, Roalfe AK, Davis RC, Davies MK, Hare R. Prognosis of all-cause heart failure and borderline left ventricular systolic dysfunction: 5 Year mortality follow-up of the Echocardiographic Heart of England Screening Study (ECHOES) Eur Heart J. 2007;28:1128–1134. doi: 10.1093/eurheartj/ehm102. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. doi: 10.1161/01.CIR.0000085166.44904.79. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study. Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 15.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 16.Furberg CD, Manolio TA, Psaty BM, Bild DE, Borhani NO, Newman A, Tabatznik B, Rautaharju PM. Major electrocardiographic abnormalities in persons aged 65 years and older (the Cardiovascular Health Study): Cardiovascular Health Study Collaborative Research Group. Am J Cardiol. 1992;69:1329–1335. doi: 10.1016/0002-9149(92)91231-r. [DOI] [PubMed] [Google Scholar]
- 17.Rautaharju PM, Manolio TA, Siscovick D, Zhou SH, Gardin JM, Kronmal R, Furberg CD, Borhani NO, Newman A. Utility of new electrocardiographic models for left ventricular mass in older adults: the Cardiovascular Health Study Collaborative Research Group. Hypertension. 1996;28:8–15. doi: 10.1161/01.hyp.28.1.8. [DOI] [PubMed] [Google Scholar]
- 18.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 19.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O'Leary D, Manolio TA. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly: the Cardiovascular Health Study. Circulation. 1995;91:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 20.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 21.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 22.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]


