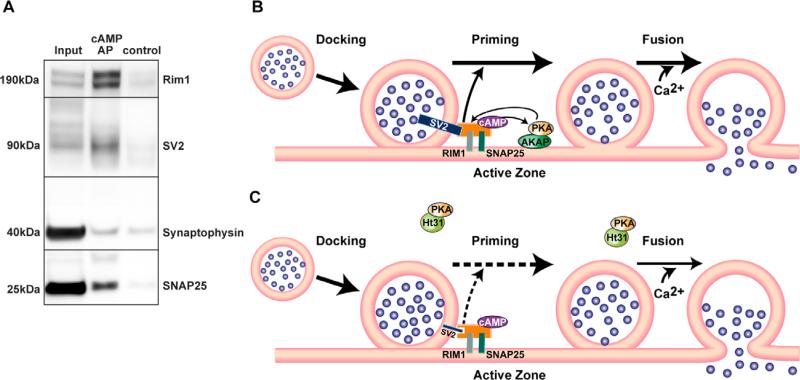
Figure 3.
SV2, Rim1, and SNAP25 co-affinity precipitate with cAMP, and presynaptically compartmentalized PKA may regulate the readily releasable pool of synaptic vesicles through SV2. A. cAMP affinity-precipitation (AP) was performed using Rp-8-AHA-cAMP-agarose beads. Under control conditions, excessive amount of cAMP was added to compete against Rp-8-AHA-cAMP-agarose beads. cAMP affinity-precipitates of hippocampal extracts showed enrichment of Rim1, SV2, and SNAP25. The synaptophysin band in the AP lane was non-specific. B. A-kinase anchoring proteins (AKAPs) maintain a pool of PKA in close proximity to a cAMP-bound protein complex that bridges between a synaptic vesicle protein SV2 and active zone proteins, including Rim1 and SNAP25. This spatial compartmentalization allows fast coupling of cAMP-PKA signaling with the priming of the docked vesicles, which increases the size of the readily releasable pool of synaptic vesicles. Ca2+-influx following neuronal activation triggers fast fusion of the primed synaptic vesicles, and the large size of the readily releasable pool of synaptic vesicles sustains long-lasting synaptic plasticity. C. When PKA anchoring is disrupted by Ht31, PKA is sequestered away from the active zone. The free PKA is unable to coordinate interactions among proteins involved in vesicle exocytosis, which results in the reduction of SV2 protein levels. In addition, AKAPs may serve as the cAMP-bound complex, and Ht31 treatment disrupts the complex, thereby reducing SV2 levels. The reduced protein levels of SV2 destabilize the link between the docked synaptic vesicles and the active zone, thus the priming of the docked vesicles is attenuated. The resulting smaller size of the readily releasable pool of synaptic vesicles causes faster depletion of the vesicle pool during neuronal activity. Therefore, long-lasting synaptic plasticity is prevented. Note that this model focuses on how presynaptically compartmentalized PKA regulates the size of the readily releasable pool and does not exclude the potential involvement of other presynaptic proteins such as synapsin1 in the priming process.