Abstract
Purpose
One potential strategy for improving voiding diary completion rates and data quality is use of a mobile electronic format. We evaluated the acceptability and feasibility of mobile voiding diaries for patients with nonneurogenic lower urinary tract dysfunction, and compared mobile and paper voiding diaries.
Materials and Methods
We prospectively enrolled children presenting with daytime symptoms of lower urinary tract dysfunction between July 2012 and April 2013. We enrolled an initial cohort of patients who were provided a paper voiding diary and a subsequent cohort who were provided a mobile voiding diary. We conducted in person interviews and assessed completion rates and quality, comparing paper and mobile voiding diary groups.
Results
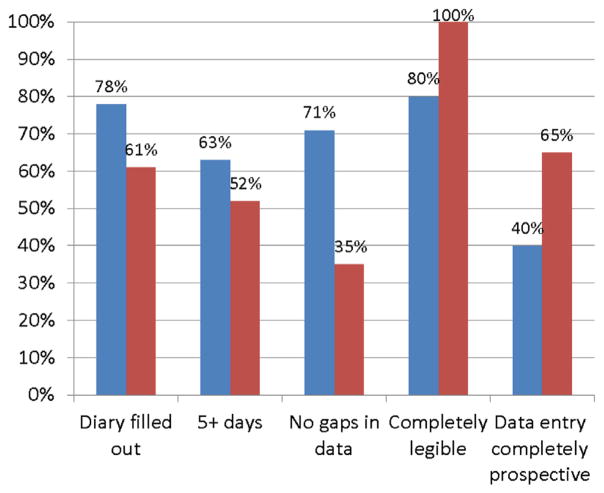
We enrolled 45 patients who received a paper voiding diary and 38 who received a mobile voiding diary. Completion rates were 78% for paper voiding diaries and 61% for mobile voiding diaries (p = 0.10). Data quality measures for patients completing paper vs mobile voiding diaries revealed a larger proportion (63% vs 52%) providing a full 5 days of data and a smaller proportion (20% vs 65%) with data gaps. However, the paper voiding diary also demonstrated a lower proportion (80% vs 100%) that was completely legible and a lower proportion (40% vs 65%) with completely prospective data entry.
Conclusions
The use of a mobile voiding diary was acceptable and feasible for our patients with lower urinary tract dysfunction, although completion rates were somewhat lower compared to paper voiding diaries. Data quality was not clearly better for either version. The mobile voiding diary format may offer data quality advantages for select groups but it did not display significant superiority when provided universally.
Keywords: defecation, lower urinary tract symptoms, mobile applications, urination
Lower urinary tract dysfunction is one of the most common conditions seen at pediatric urology clinics. Although the exact prevalence of lower urinary tract dysfunction is difficult to determine, the prevalence of associated conditions including urinary incontinence and urgency ranges from 7.8% to 21.8% in school-age children.1–3 Furthermore, urinary incontinence represents 40% of pediatric urology outpatient referrals.4 The treatments for patients with lower urinary tract dysfunction are largely behavioral, with standard recommendations involving assessment of elimination patterns via a combined bladder and bowel voiding diary. The voiding diary has traditionally been viewed as an integral component of the evaluation and management of pediatric voiding issues.5–7 Data are usually recorded in a paper voiding diary format and are often incomplete, and the quality is usually poor.8
One potential method to achieve the goal of a complete, high quality VD for each patient is to incorporate the use of a software application to collect the data via a mobile device. Previous research regarding mobile technology for patient data tracking suggests it is a viable tool for influencing behavior change in conditions such as obesity.9,10 However, there have been no previous rigorous investigations evaluating the development and use of such tools for recording voiding habits in children. The aims of our study were to 1) develop a mobile voiding diary Web based software application, 2) evaluate the acceptability and feasibility of the mobile voiding diary for patients with nonneurogenic voiding dysfunction, and 3) compare the mobile voiding diary and the standard paper voiding diary.
METHODS
We conducted an institutional review board approved study of pediatric patients with nonneurogenic LUT dysfunction who were asked to fill out a VD as part of the initial evaluation. Using a pre/post design, we compared a cohort of patients who were provided a standard pVD before the clinic visit with a subsequent cohort who were provided a link to an mVD Webapp. Our primary outcome was diary completion rate. A power calculation revealed that we needed to enroll 38 patients in each group to detect a 30% difference in completion rates between the groups.
Development of mVD Webapp
In conjunction with software developers at our institution we designed an mVD Webapp for recording urinary and bowel habits (fig. 1). The application was programmed on a flexible software platform that allowed for use on any mobile device with an Internet browser. After loading the Webapp onto a mobile device patients entered their voiding inputs in real time. These inputs were then automatically uploaded and stored on a secure research server in real time, and could be downloaded into a PDF file that was structurally similar to the pVD that clinicians at our institution are accustomed to reviewing.
Figure 1.
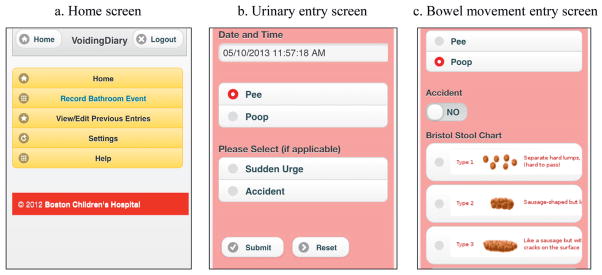
Mobile voiding diary
Patient Population
Patients with nonneurogenic LUT dysfunction who are referred to the urology department at our institution are generally treated at the VIP clinic. More than 3,000 patients are evaluated yearly at the clinic, and each patient undergoes a standardized evaluation protocol that includes renal ultrasound, abdominal x-ray, measurement of urinary flow rate, measurement of post-void residual, and recording of bowel and bladder elimination habits in a pVD. The pVD is provided in a new patient packet that we ask families to completle before their visit. Families are specifically asked to fill out the pVD for a total of 5 days.
For this study we prospectively enrolled pediatric patients 4 to 17 years old presenting to the VIP clinic for the initial visit between July 2012 and April 2013. We excluded patients who were non-English speaking, had no mobile Internet access, had a neurogenic cause of LUT dysfunction, were on intermittent catheterization, had an active urinary tract infection, had nighttime symptoms only or were never asked to fill out a diary. Families were provided with a parking coupon and a $10 gift card for participation in the study.
Prospective Data Collection
We recruited patients and families who received a pVD and who met eligibility criteria by approaching them in person at their clinic visit. For those patients who provided consent to participate, we conducted in person interviews assessing the domains of VD completion rates, data quality, patient/parent attitudes toward the diary and access to the Internet at home. For a qualitative assessment we asked all participants open-ended questions, including, “How could we have made it easier for you and your family to fill out the voiding diary?” For this pVD group we also assessed the acceptability of a hypothetical mVD. The full pVD survey instrument is provided in Appendix 1 (http://jurology.com/). Through chart review and review of the diaries we also evaluated patient demographics, presenting complaint(s) and additional data quality measures, including number of days the diary was filled out, presence or absence of gaps in the data, legibility and recording of urine, stool or both.
After recruitment was complete for patients receiving a pVD we then began providing a link to the mVD in our new patient packets to all patients with mobile Internet access. We then recruited a cohort of patients who received the mVD link. For those patients who elected to participate we collected data similarly to those receiving a pVD. We also assessed the overall acceptability of the mVD format and the amount of data transferred from paper to the mVD Webapp, and elicited feedback regarding technical difficulties and suggestions for improvement of future versions of the mVD (Appendix 2, http://jurology.com/).
Statistical Analysis
Descriptive statistics were used to characterize the population of new patients presenting to our VIP clinic who participated in the study. Demographics and presenting complaints were compared between the pVD and mVD groups. Completion rates and data quality measures were compared between patients receiving paper vs mobile VDs using Fisher exact testing. Parental perspectives regarding the VD were compared for patients who completed the VD vs those who did not, also using Fisher exact testing. We stratified these results by VD format (mobile vs paper).
RESULTS
We enrolled 45 patients (median age 6.5 years, 69% female) who received a pVD, and 38 patients (7 years, 68% female) who received an mVD. Patient demographics and presenting complaints are detailed in table 1.
Table 1.
Patient characteristics
| Paper Diary | Mobile Diary | |
|---|---|---|
| Median yrs age (IQR) | 7 (5–9.5) | 6.5 (5–9) |
| No. gender/total No. (%) | ||
| Male | 14/45 (31.1) | 12/38 (31.6) |
| Female | 31/45 (68.9) | 26/38 (68.4) |
| No. chief complaint/total No. (%):* | ||
| Nocturnal enuresis | 3/45 (6.7) | 7/38 (18.4) |
| Daytime enuresis | 6/45 (13.3) | 4/38 (10.5) |
| Day + night wetting | 26/45 (57.8) | 15/38 (39.5) |
| Urinary frequency | 11/45 (24.4) | 12/38 (31.6) |
| Urinary urgency | 13/45 (28.9) | 4/38 (10.5) |
| Dysuria | 2/45 (4.4) | 2/38 (5.3) |
| Other† | 7/45 (15.6) | 9/38 (23.7) |
Totals greater than 100% are due to patients presenting with multiple chief complaints.
Includes urinary tract infections (febrile and nonfebrile), constipation, infrequent voiding, incomplete bladder emptying and urinary leakage.
Diary completion rates and data quality measures are illustrated in figure 2. Overall we observed diary completion rates of 78% (35 of 45) for pVDs and 61% (23 of 38) for mVDs (p = 0.10). Of the 23 patients in the mVD group 18 (78%) used a smartphone to complete the diary and the remainder used a tablet computer. Two patients (5.7%) in the pVD group reported filling out the diary but forgetting to bring the paper to the clinic. Data quality measures for patients completing pVDs (vs mVDs) demonstrated a larger proportion of patients filling out a full 5 days of data (63% paper vs 52% mobile, p = 0.27) and a smaller proportion with gaps in the data (20% vs 65%, p <0.001). However, the pVD also showed a lower proportion that was completely legible (80% vs 100%, p = 0.13), and a lower proportion where all data were entered prospectively (40% vs 65%, p = 0.06).
Figure 2.
Completion rates and data quality for paper (blue bars) vs mobile (red bars) VD.
Overall the child participated in filling out the voiding diary in 57% of cases. There were no differences in child vs parent participation between the pVD and mVD groups (table 2, p = 0.52). The youngest child who participated in filling out a pVD was 4 years old, and the youngest who participated in filling out an mVD was 5. Although there were no children older than 13 years in the pVD group, all 3 patients in the mVD group who were older than 13 (1 was 14 and 2 were 17) completely filled out the mVD on their own devices without any parental input. The majority of patients in both groups reported that the diary was not difficult to complete, rating the ease as 4 or 5 on a 5-point scale (p = 0.60 for paper vs mobile).
Table 2.
Patient/parental perspective of voiding diary
| Paper Diary | Mobile Diary | p Value | |
|---|---|---|---|
| No. completing VD/total No. (%) | 0.52 | ||
| Parent | 18/35 (51.4) | 15/23 (65.2) | |
| Child | 3/35 (8.6) | 3/23 (13.0) | |
| Both | 12/35 (34.3) | 5/23 (21.7) | |
| Other* | 2/35 (5.7) | 0/23 (0) | |
| No. difficulty completing VD/total No. (%): | 0.60 | ||
| 1—Impossible | 0/35 (0) | 0/23 (0) | |
| 2 | 1/35 (2.9) | 2/23 (8.7) | |
| 3 | 3/35 (8.6) | 3/23 (13.0) | |
| 4 | 17/35 (48.6) | 12/23 (52.2) | |
| 5—No trouble at all | 14/35 (40.0) | 6/23 (26.1) |
Grandmother, aunt.
Parental attitudes toward the voiding diary are detailed in table 3. When comparing diary completers to noncompleters in both groups, we noted that parents who did not complete the diary were more likely to feel that the diary was too long and/or inconvenient. Parents in this group were also more likely to be unsure of the voiding habits of their child.
Table 3.
Parental response to voiding diary
| Paper Diary | Mobile Diary | |||||
|---|---|---|---|---|---|---|
| No. Completers/Total No. (%) | No. Noncompleters/Total No. (%) | p Value | No. Completers/Total No. (%) | No. Noncompleters/Total No. (%) | p Value | |
| Forgot to complete VD | 3/35 (8.6) | 6/8 (75) | <0.001 | 2/22 (9.1) | 10/14 (71.4) | <0.001 |
| Felt VD was inconvenient | 5/35 (14.3) | 4/5 (80) | 0.006 | 1/22 (4.5) | 8/14 (57.1) | <0.001 |
| Felt VD was too long | 3/35 (8.6) | 3/6 (50) | 0.03 | 1/23 (4.3) | 4/12 (33.3) | 0.04 |
| Did not understand directions | 1/35 (2.9) | 0/7 (0) | >0.99 | 0/22 (0) | 3/11 (27.3) | 0.03 |
| Unsure of voiding habits of child | 10/35 (28.6) | 5/6 (83.3) | 0.02 | 2/21 (9.5) | 4/12 (33.3) | 0.16 |
| Did not think VD was important | 1/35 (2.9) | 0/7 (0) | >0.99 | 1/23 (4.3) | 1/12 (8.3) | >0.99 |
| Thought VD could help child | 35/35 (100) | 5/7 (71.4) | 0.02 | 22/22 (100) | 11/13 (84.6) | 0.13 |
| Completed but forgot to bring VD | 2/35 (5.7) | 1/6 (16.7) | 0.39 | 0/23 (0) | 0/15 (0) | >0.99 |
Qualitative feedback was generated from parents and patients in the pVD and mVD groups. Most of the feedback from parents in the pVD group was regarding formatting. For example several families from the pVD group felt that we should include more time slots, and multiple families mentioned a mobile and/or electronic option as a general suggestion for improvement. Additionally 87% of families were interested in a mobile/electronic VD option when directly queried. Families completing the mVD provided our team with concrete technical suggestions that allowed iterative improvement of our design by adding a free text notes field to our application. Although the design/formatting feedback for the mVD was largely positive, several families experienced technical difficulties that necessitated support from our research and software programming team members.
DISCUSSION
For this study we developed and then systematically evaluated the use of an mVD for patients with lower urinary tract dysfunction presenting to our high volume VIP clinic. Although we found the mVD format to be generally well received by patients and families, our completion rates were modestly lower for the mVD compared to paper, and we encountered data quality issues with both formats.
The concept of an electronic voiding diary is not new. An electronic VD, Compu-Void, was developed and piloted in the early 1990s by Rabin et al.11,12 Although the initial study results were promising, the generalizability of this system was limited due to the device being a cumbersome computerized unit with only 1 function. The practical applicability of such a format has increased substantially now that smartphones and tablet computers are becoming ubiquitous in our society.
There are currently multiple commercially available mVDs for adults and children.13–15 Although electronic voiding diaries have been evaluated in the research setting in adults,12,16 to our knowledge this is the first investigation that compares the use of mobile technology with the standard pVD in children with LUT dysfunction.
One recent study investigating the use of a Palm based (Palm, Inc., Sunnyvale, California) VD in adults with overactive bladder revealed promising results. In a crossover study of 35 patients Quinn et al found that the median number of incontinent episodes per day was equivalent between the paper and electronic diaries.16 However, there were fewer daily voids and fewer urgency episodes recorded in the pVD, suggesting that the mVD may be more sensitive for recording parameters such as frequency and urgency.
The use of mobile electronic tracking and data entry systems has been studied even more extensively in fields outside urology, and the results have been mixed. Researchers have examined electronic diaries for a wide range of conditions, including chronic pain, asthma and gastrointestinal symptoms. While nearly all studies demonstrate the feasibility of an electronic format for recording biometric data and patient symptoms,17–22 it is less clear whether an electronic diary format alone is truly superior to the traditional paper and pencil approach.
Electronic diaries have been associated with higher compliance rates in children and adults with chronic pain,23,24 and a study of infant behavior monitoring suggested that electronic diaries may facilitate more frequent behavior monitoring compared to paper diaries.25 However, several studies of children and adults with asthma have shown decreased compliance and reliability of data in those completing a mobile diary compared to a paper diary.26,27 Additionally a study of parental report of infant sleep habits suggested poorer compliance in patients completing an electronic version compared to a paper version.28
A notable difference between previous investigations and our study is that the mVD provided to our patients was loaded onto an existing device, whereas previous electronic diary studies have examined the use of a separate device designed specifically for symptom monitoring. We speculate that the use of a device already owned by the patient or parent may have a positive effect on diary completion, particularly for participants who are comfortable using smartphones or tablet computers for a variety of daily functions. With the ubiquity of medically oriented tracking applications, we anticipate that the usefulness of mobile application based patient tracking will be further clarified in future literature.
Although studies in the urological and non-urological literature have suggested that the use of mobile software for tracking symptoms may have advantages in terms of compliance and data quality, our study did not reveal a clear advantage of the mVD for our patient population. Thus, our team has discussed several strategies for improving VD completion rates going forward. It is noteworthy that for the purposes of this study we provided the mVD to all patients with a smartphone or tablet computer. We postulate that completion rates could be improved by offering patients a choice between paper and mobile VDs rather than making one or the other the default, and we are currently using this strategy at our clinic. This point could be particularly relevant for individuals who own smartphones but who are not particularly comfortable with using many of the features of the phone. Additionally future versions of an mVD might incorporate incentives such as gamification or a reminder system to encourage compliance with data entry. Previous studies have suggested that reminders such as programmed alarms can improve compliance with data entry.16,23 We purposely did not program these features into our initial version of the VD since we specifically aimed to determine whether simply using a mobile system would improve completion rates and data quality.
Notable strengths of our study include its prospective design, inclusion of a control group and the use of direct, in person detailed queries of patients and parents regarding their experience and opinions. Limitations include that this was a single institution study with a relatively small sample size. It remains to be determined whether these results are applicable within other settings or patient populations. For feasibility reasons we elected a pre/post design, not a true randomization. Thus, other unmeasured factors might have influenced our results if such factors changed during the study period. Additionally there were no teenage patients in the pVD group, limiting the conclusions that we could draw regarding our adolescent patients. We also did not examine whether patients who are smartphone naive could use the mobile application. Finally, we purposely did not incentivize completion (as discussed previously), which could have improved completion rates in both groups.
CONCLUSIONS
The development and use of a mobile voiding diary was acceptable and feasible for our population of patients with LUT dysfunction, although completion rates were somewhat lower than with paper voiding diaries. Data quality was not clearly better for either version. Patient feedback was integral in the development of updated versions of our mVD. The mVD format may offer data quality advantages for certain patients but did not demonstrate significant superiority when provided to all patients at our clinic with access to a smartphone.
Acknowledgments
Supported by Boston Children’s Hospital O’Brien Center Pilot and Feasibility Grant 5P50DK065298-10.
Pamela Kelly, Sara Walsh and Erin Hennessy provided support and advice during study planning and data collection. Graciela Rivera Castro and Marian Younge assisted with patient and family interviews.
Abbreviations and Acronyms
- LUT
lower urinary tract
- mVD
mobile voiding diary
- pVD
paper voiding diary
- VD
voiding diary
- VIP
voiding improvement program
- Webapp
Web based software application
References
- 1.Sureshkumar P, Craig JC, Roy LP, et al. Daytime urinary incontinence in primary school children: a population-based survey. J Pediatr. 2000;137:814. doi: 10.1067/mpd.2000.109196. [DOI] [PubMed] [Google Scholar]
- 2.von Gontard A, Heron J, Joinson C. Family history of nocturnal enuresis and urinary incontinence: results from a large epidemiological study. J Urol. 2011;185:2303. doi: 10.1016/j.juro.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Vaz GT, Vasconcelos MM, Oliveira EA, et al. Prevalence of lower urinary tract symptoms in school-age children. Pediatr Nephrol. 2012;27:597. doi: 10.1007/s00467-011-2028-1. [DOI] [PubMed] [Google Scholar]
- 4.Rushton HG. Wetting and functional voiding disorders. Urol Clin North Am. 1995;22:75. [PubMed] [Google Scholar]
- 5.De Gennaro M, Niero M, Capitanucci ML, et al. Validity of the International Consultation on Incontinence Questionnaire-Pediatric Lower Urinary Tract Symptoms: a screening questionnaire for children. J Urol. 2010;184:1662. doi: 10.1016/j.juro.2010.03.075. suppl. [DOI] [PubMed] [Google Scholar]
- 6.Kwak KW, Park KH. Clinical inconsistency of lower urinary tract symptoms between questionnaire and bladder diary in children with nocturnal enuresis. J Urol. 2008;180:1085. doi: 10.1016/j.juro.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 7.Nevéus T, von Gontard A, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children’s Continence Society. J Urol. 2006;176:314. doi: 10.1016/S0022-5347(06)00305-3. [DOI] [PubMed] [Google Scholar]
- 8.Tincello DG, Williams KS, Joshi M, et al. Urinary diaries: a comparison of data collected for three days versus seven days. Obstet Gynecol. 2007;109:277. doi: 10.1097/01.AOG.0000252832.21986.c8. [DOI] [PubMed] [Google Scholar]
- 9.Gerber BS, Stolley MR, Thompson AL, et al. Mobile phone text messaging to promote healthy behaviors and weight loss maintenance: a feasibility study. Health Informatics J. 2009;15:17. doi: 10.1177/1460458208099865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stephens J, Allen J. Mobile phone interventions to increase physical activity and reduce weight: a systematic review. J Cardiovasc Nurs. 2013;28:320. doi: 10.1097/JCN.0b013e318250a3e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabin JM, McNett J, Badlani GH. Computerized voiding diary. Neurourol Urodyn. 1993;12:541. doi: 10.1002/nau.1930120604. [DOI] [PubMed] [Google Scholar]
- 12.Rabin JM, McNett J, Badlani GH. A computerized voiding diary. J Reprod Med. 1996;41:801. [PubMed] [Google Scholar]
- 13. [Accessed January 21, 2014];Bladder Diary. Available at https://itunes.apple.com/us/app/bladder-diary/id361552315?mt=8.
- 14. [Accessed January 21, 2014];Bladder Pal. Available at https://play.google.com/store/apps/details?id=com.quarkstudios.bladderpal.
- 15. [Accessed January 21, 2014];HapPee Time. Available at https://itunes.apple.com/us/app/happee-time/id622586272?mt=8.
- 16.Quinn P, Goka J, Richardson H. Assessment of an electronic daily diary in patients with overactive bladder. BJU Int. 2003;91:647. doi: 10.1046/j.1464-410x.2003.04168.x. [DOI] [PubMed] [Google Scholar]
- 17.Allena M, Cuzzoni MG, Tassorelli C, et al. An electronic diary on a Palm device for headache monitoring: a preliminary experience. J Headache Pain. 2012;13:537. doi: 10.1007/s10194-012-0473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowienczyk PJ, Lawson CP, Morris J, et al. Electronic diary to record physiological measurements. Lancet. 1992;339:251. doi: 10.1016/0140-6736(92)90061-7. [DOI] [PubMed] [Google Scholar]
- 19.Kajander K, Latti M, Hatakka K, et al. An electronic diary versus a paper diary in measuring gastrointestinal symptoms. Dig Liver Dis. 2007;39:288. doi: 10.1016/j.dld.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Kupczyk M, Haque S, Sterk PJ, et al. Detection of exacerbations in asthma based on electronic diary data: results from the 1-year prospective BIOAIR study. Thorax. 2013;68:611. doi: 10.1136/thoraxjnl-2012-201815. [DOI] [PubMed] [Google Scholar]
- 21.Lewis B, Lewis D, Cumming G. Frequent measurement of chronic pain: an electronic diary and empirical findings. Pain. 1995;60:341. doi: 10.1016/0304-3959(94)00143-3. [DOI] [PubMed] [Google Scholar]
- 22.Litcher-Kelly L, Kellerman Q, Hanauer SB, et al. Feasibility and utility of an electronic diary to assess self-report symptoms in patients with inflammatory bowel disease. Ann Behav Med. 2007;33:207. doi: 10.1007/BF02879902. [DOI] [PubMed] [Google Scholar]
- 23.Stone AA, Shiffman S, Schwartz JE, et al. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003;24:182. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 24.Palermo TM, Valenzuela D, Stork PP. A randomized trial of electronic versus paper pain diaries in children: impact on compliance, accuracy, and acceptability. Pain. 2004;107:213. doi: 10.1016/j.pain.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Lam J, Barr RG, Catherine N, et al. Electronic and paper diary recording of infant and caregiver behaviors. J Dev Behav Pediatr. 2010;31:685. doi: 10.1097/DBP.0b013e3181e416ae. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer EO, Kelley N, Hovell MF. Randomized, cross-over evaluation of mobile phone vs paper diary in subjects with mild to moderate persistent asthma. Open Respir Med J. 2008;2:72. doi: 10.2174/1874306400802010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ireland AM, Wiklund I, Hsieh R, et al. An electronic diary is shown to be more reliable than a paper diary: results from a randomized crossover study in patients with persistent asthma. J Asthma. 2012;49:952. doi: 10.3109/02770903.2012.724754. [DOI] [PubMed] [Google Scholar]
- 28.Muller S, Hemmi MH, Wilhelm FH, et al. Parental report of infant sleep behavior by electronic versus paper-and-pencil diaries, and their relationship to actigraphic sleep measurement. J Sleep Res. 2011;20:598. doi: 10.1111/j.1365-2869.2011.00926.x. [DOI] [PubMed] [Google Scholar]