Abstract
Background
Nerves are often enlarged in chronic inflammatory demyelinating polyneuropathy (CIDP). We studied changes with treatment over time.
Methods
We retrospectively compared serial ultrasound measurements of median and ulnar nerve size with clinical and electrodiagnostic evaluations in 23 CIDP subjects. We defined remission as stable clinical improvement on low or decreasing amounts of medication.
Results
Nerves were normal at last follow-up more often in subjects who achieved remission than those who did not (10/13 vs. 0/10, P=0.0001). Nerves were normal or smaller (>30% reduction) more often in subjects whose grip strength improved or remained strong than those who weakened (12/16 vs. 0/3, P=0.04) and in subjects whose demyelinating electrodiagnostic features resolved than those whose demyelination persisted (7/7 vs. 6/12, P=0.04). Over time, nerve size decreased more in subjects with baseline nerve enlargement who achieved remission than those who did not (−41% vs. 7%, P=0.04).
Conclusion
In CIDP, enlarged nerves normalized or decreased with remission.
Keywords: ultrasound, nerve, chronic inflammatory demyelinating polyneuropathy (CIDP), treatment, longitudinal
Introduction
Ultrasound is a painless, inexpensive technique that can detect nerve pathology at the bedside. In mononeuropathy, ultrasound is a sensitive technique to detect focal nerve pathology1 and complements the electrodiagnostic evaluation of nerve entrapment and traumatic neuropathies2–7. In polyneuropathies, nerve enlargement is frequently detected in patients with inherited and acquired demyelinating peripheral polyneuropathies8–13. In chronic inflammatory demyelinating polyneuropathy (CIDP), nerve enlargement has been reported in 69–100% of patients9,14–16 and could reflect disease activity. The pattern and degree of nerve enlargement in CIDP varies with disease duration and differs from other acquired and inherited demyelinating neuropathies16–18. CIDP subjects with more nerve enlargement have slower motor conduction velocities9 and more weakness16,19. Nerve enlargement in CIDP may attenuate with early treatment. Subjects with CIDP treated within 3 months of symptom onset have less enlarged nerves than those with longer disease duration before treatment18.
It is not known how nerve size in CIDP changes with treatment over time. Most prior studies of nerve size in acquired demyelinating neuropathies have been cross-sectional. A single case report in a child with Guillain-Barré syndrome reported improvement in nerve enlargement over time8, suggesting that in some cases nerve enlargement is reversible. However, nerve enlargement in CIDP and Guillain-Barré syndrome can persist despite treatment and clinical recovery9,18,20. To determine how nerve size changes with treatment and over time in CIDP, we performed a retrospective review of serial clinical, electrodiagnostic, and nerve ultrasound results in a series of subjects with CIDP.
Methods
This retrospective study was approved by the Washington University institutional review board. Results are reported as median (range) unless specified. We identified 23 subjects (Table 1) with clinical and nerve conduction features that met diagnostic criteria for CIDP21 and who had 2 or more nerve ultrasound examinations of unilateral median and ulnar nerves identified from our database of patients evaluated between June 2007 and July 2013. The right or left arm was selected to coincide with the electrodiagnostic study if known at the time of the ultrasound; otherwise we imaged the arm reported by the patient as the most severely affected. Five subjects were treatment naïve. All patients were followed in our neuromuscular clinic by neuromuscular neurologists, and charts were reviewed to determine disease course and response to therapy. Disease duration was defined as the time from symptom onset to the ultrasound study.
Table 1.
Baseline Characterstics (median (range))
| N= | Age (years) |
Men/ Women |
Height (cm) |
Weight (kg) |
Disease Duration at Baseline (months) |
Months Between Initial and Final Ultrasound |
|---|---|---|---|---|---|---|
| 23 | 50 (7–78) | 14/9 | 175 (122–191) | 91 (22–147) | 21 (0.7–252) | 24 (4–70) |
Clinical Assessment
Neurologic examination was performed by the treating attending neuromuscular neurologist during the patients’ clinical visits as part of the standard of care and prior to the electrodiagnostic or ultrasound examination. In our neuromuscular clinic, strength is measured typically using hand-held dynamometry. Grip strength was the most routinely repeated strength measurement identified during the chart review. Grip strength was measured in pounds-force by the treating physician with a hand-held grip dynamometer in the imaged arm of 21 subjects, and it was repeated in 19 subjects 7 (1–32) months later. Minimum grip strength (≤3/5) was recorded as 1 pound-force (4 Newton). All subjects had neurologic symptoms/signs affecting the imaged arm at the time of the baseline ultrasound exam except 3 whose symptoms in the imaged arm had previously resolved with treatment. The results of the ultrasound examination were not available to the examining physician at the time of the clinical or electrodiagnostic evaluation.
Electrodiagnostic Assessment
Electrodiagnostic testing was performed in our neurophysiology laboratory using standard techniques as requested by the attending physician as standard of care and interpreted by a neuromuscular physician with expertise in electrodiagnostics22. Typically, electrodiagnostic evaluation includes the median, ulnar, tibial, and fibular nerves. Electrodiagnostic studies were tailored for each patient, and some variability occurred. All subjects had electrodiagnostic testing with demyelinating features (onset latencies/ conduction velocities in the demyelinating range, increased temporal dispersion, or conduction block)21 at the time of diagnosis, often prior to the initial ultrasound study. Electrodiagnostic testing during the ultrasound study period was available in 20 subjects and was performed concurrently with the ultrasound examination in 29 studies. Nine subjects had repeat electrodiagnostic testing during the study 7 (2–27) months later. To assess for interval changes in the remaining 11 subjects, we included comparisons to electrodiagnostic studies performed prior to the initial ultrasound examination. Interval changes in electrodiagnostic results (worsened, stable, or improved) and the continued presence of demyelinating features were determined based on the interpretation of the electrophysiologist and without knowledge of the ultrasound findings. If no demyelinating features were present on the most recent electrodiagnostic study, we categorized the demyelination as resolved.
Ultrasound Assessment
Ultrasound examinations were performed using a Philips HD11XE or iu22 imaging system with an L12-5 linear array probe. Median and ulnar nerves were chosen for study, as they are easily imaged at several sites along their length. One investigator (CMZ) obtained all ultrasound images. The ultrasound probe was kept perpendicular to the nerve by maintaining an angle at which the ultrasound image of the nerve appeared smallest and brightest. Nerve cross-sectional areas (NCSA) were measured by tracing nerves just inside their hyperechoic rims. Three separate NCSA measurements, with the probe repositioned for each measurement, were averaged at each nerve site. Nerve vascularity was not assessed routinely. Most subjects were seated with the entire arm extended anteriorly, supinated, and supported by a pillow on a table at approximately mid-thoracic height. Hospitalized subjects were examined in the supine position with the arm supinated, abducted, and supported at body level. Each patient had transverse images obtained from 4 nerve sites: the proximal and distal median and ulnar nerves, avoiding sites of possible entrapment, approximately 2/3 of the distance from the lateral tip of the acromion to the lateral epicondyle of the humerus (proximal) and in the forearm, approximately ¾ of the distance from the medial epicondyle of the humerus to the ulnar styloid process (distal). This imaging protocol can usually be completed in less than 20 minutes.
To allow direct comparisons between patients of different heights, a “nerve size index” (NSI), with size measurements corrected for height, was calculated by comparing the measured NCSA to the expected NCSA based on height for each nerve site and subject9. The NSI was derived from the slope (m) and y intercept (b) of the simple linear regression line that related the nerve cross sectional area to height using the equation: [NCSA (mm2) / {(m × height (cm)} + b] * 100%. Corrections for height (m) are + 0.041 mm2/cm for the ulnar nerve in the arm and forearm and 0.054 mm2/cm for the median nerve in the arm and forearm. Constants (b) were −1.5 for the ulnar and median nerve at the forearm and arm. Average NSI values were calculated for each subject averaging the proximal and distal median and ulnar NSI measurements.
Data Analysis
Nerve enlargement was defined as an average NSI greater than 2 standard deviations above the mean NSI in controls (>132%)9. We defined a significant (α=0.05) change in nerve size as >30% difference from baseline based on our laboratory upper limit of normal, derived from 28 ultrasound examinations in 13 healthy controls repeated over an average of 15 months. We defined a change in grip strength based on the distribution of our results and to reflect a clinical meaningful change as >25% difference from baseline. In subjects with more than 1 repeat measure, the largest change from baseline is reported. Groups were compared using the Fisher exact, Mann-Whitney U, related samples Wilcoxson signed ranks or McNemar tests. Correlations were assessed with Spearman rank correlation coefficients (rs).
The clinical course between ultrasound examinations was categorized as in remission or as treatment dependent based on the treating clinician impression and included the subject’s subjective complaints, physical examination, and decisions to modify therapy. Remission was defined as stable clinical improvement that persisted with reductions in medication or, in 1 (#4, age 7 years), as continued improvement on stable, low dose, treatment without attempts at dose reduction. Treatment dependence was defined as requiring increasing or chronic medication with symptom recurrence or relapse with attempts at dose reduction.
Results
Baseline Characteristics
Seventeen (74%) subjects had enlarged nerves at baseline (Supplemental Table1). Two were treatment naïve, 3 had a single prior treatment with IVIG or plasma exchange for presumed Guillain-Barré syndrome, 9 were on treatment, 1 had relapsed after weaning off treatment, and 2 were stable off treatment for 7 months and 1 year, respectively. Six (26%) subjects had normal nerves at baseline. Three were treatment naïve, 1 had a single prior treatment of IVIG, and 2 were steroid responsive and tolerating a stable low dose or reduction in medications without recurrence of symptoms. At baseline, grip strength was 267(4 to 632) Newtons and did not correlate with average nerve size (rs= 0.1, P=0.5). At baseline, disease duration correlated with both grip strength (rs= 0.5, P=0.02) and average nerve size (rs=0.5, P=0.02).
Neither baseline age, gender, height, weight, disease duration, grip strength, number of immunomodulating medications, nor time between first and last ultrasound exams differed (P≥0.06) between those who did or did not achieve remission, have persistent demyelinating features, or have electrodiagnostic or grip strength measurements performed.
Changes over Time
Enlarged nerves (n=17) reduced in size [−32% (−174% to +62%) change] over time in most: 6 normalized [1 (#2) transiently], 5 showed smaller nerves, 5 were unchanged, and 1 enlarged further (Supplemental Table 1). Normal nerves (n=6) remained unchanged in 4, reduced by 38% in 1 (#16) whose baseline NSI was at the upper limit of normal, and enlarged in 1 (#22). Average NSI was smaller (P=0.03), grip strength was stronger (P=0.02), and demyelinating features were less common (P=0.02) at last follow up than baseline (Table 2). The improvements in nerve size, grip strength, and electrodiagnostic features generally occurred only in those who achieved remission (Table 3).
Table 2.
Summary of Repeated Assessments
| Value at: | ||||||
|---|---|---|---|---|---|---|
| Test | N= | # of tests per subject |
Months between repeat tests |
Baseline | Last Follow-Up |
Last Follow- Up vs. Baseline |
| Nerve Ultrasound: Average Nerve Size Index | 23 | 3 (2–4) | 7 (1–32) | 172% (82–461) | 137% (87–388) | P=0.03 |
| Grip Strength | 19 | 3 (2–4) | 7 (1–32) | 271N (4 – 632) | 400N (107 – 636) | P=0.02 |
| Electro-diagnostics | 20* | 2(2–3)* | Not Assessed | Demyelinating Features Present | P=0.02 | |
| N=19** | N=12 | |||||
includes 11 subjects with baseline study performed prior to initial nerve ultrasound.
Demyelinating features of motor nerve conduction studies include: conduction block, very slowed conduction velocity, very prolonged distal latency, or increased temporal dispersion.21 One subject (#23) with absent electrodiagnostic reponses was excluded.
All values median (range) unless otherwise specified.
Table 3.
Summary of Repeat Assessments by Remission
| Subject Characteristic | ||||||
|---|---|---|---|---|---|---|
| Remission | Failed Medication Taper | |||||
| Value at: | Value at: | |||||
| Test | N= | Baseline | Last Follow-Up |
N= | Baseline | Last Follow-Up |
| Nerve Ultrasound: Average Nerve Size Index | 13 | 158% (82–289) | 114%** (87–158) | 10 | 173% (111–461%) | 203% (137–388) |
| Grip Strength | 12 | 231N (4–454) | 405N** (107–476) | 7 | 311N (85–632) | 298N (245–636) |
| Electro-diagnostics | 10 | Demyelinating Features Present | 10 | Demyelinating Features Present | ||
| N=10† | N=3* | N=9† | N=9 | |||
P=0.02,
P≤0.009 compared to baseline. All values median (range) unless otherwise specified.
includes baseline studies performed prior to initial nerve ultrasound. Demyelinating features of motor nerve conduction studies include conduction block, very slowed conduction velocity, very prolonged distal latency, or increased temporal dispersion.21
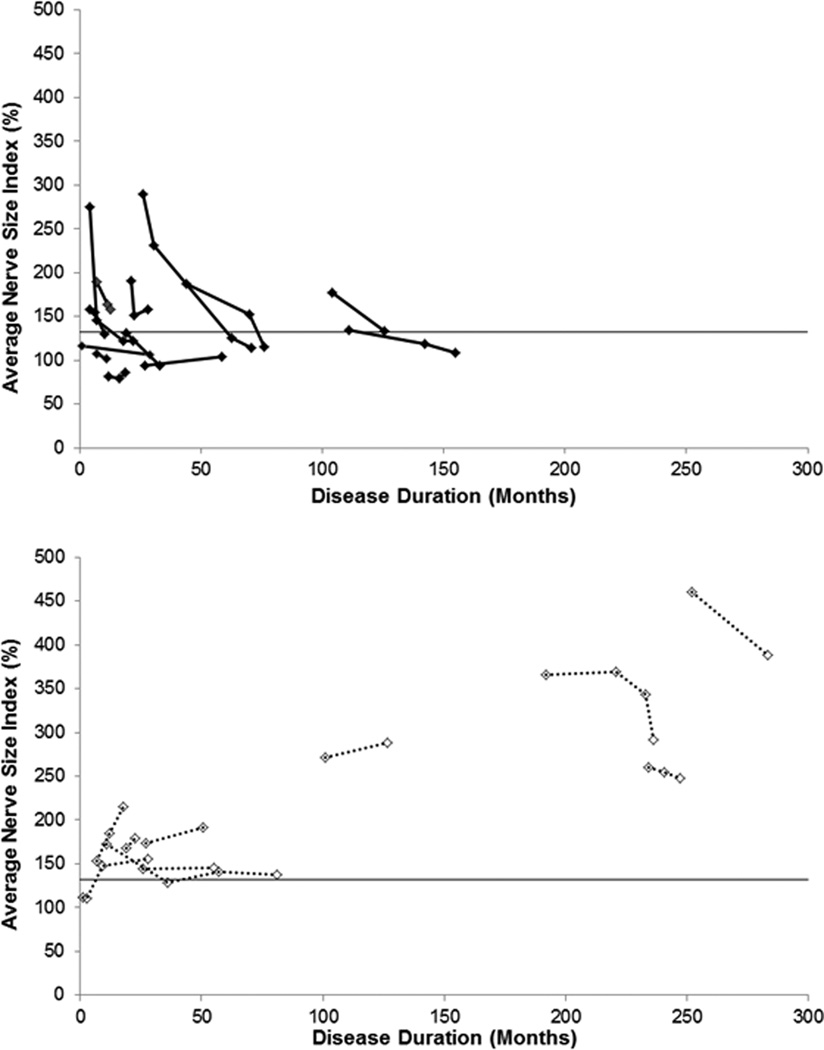
In subjects who achieved remission, nerves at follow-up were more often normal and showed greater reductions from baseline than in those who did not (Figure 1). Subjects who achieved remission were more likely (P=0.0001) to have normal nerves at last follow-up (10/13) than those who did not (0/10). Subjects with enlarged nerves at baseline who achieved remission had a greater (P=0.04) reduction in nerve size than those who did not achieve remission (−41% vs. +7 %), despite similar nerve size at baseline (P=0.8). Thirteen subjects achieved remission. At follow up, nerves were normal (n=10) or smaller (n=3) in all. Ten subjects were treatment dependent without tolerating dose reduction; all had enlarged nerves at last follow-up. Of these, 7 treatment-dependent subjects showed persistently enlarged (n=5) or enlarging (#7 and #22) nerves. Two treatment-dependent subjects (#8 and #15) showed reductions in nerve size but still had massively enlarged nerves (average NSI 389% and 292%). One (#2) had large nerves that normalized initially but then became abnormally enlarged again during a relapse.
Figure 1. Changes in Nerve Size over Time in CIDP.
Nerves of subjects who achieved remission (top panel, solid lines) reduced or normalized in size over time. In contrast, nerves of subjects who were treatment dependent (bottom panel, dotted lines) generally remained or became enlarged. The solid horizontal line is the upper limit of normal nerve size.
Subjects with resolution of demyelinating features on electrodiagnostic testing were more likely (P=0.04) to have normal or smaller nerves than those with persistent demyelinating features. Seven subjects had resolution of demyelinating features; all showed normal (n=5) or smaller (n=2) nerves at follow up. Twelve subjects had persistent demyelinating features, only half (6/12) showed normal or smaller nerves at follow-up. Four subjects (# 7, 12, 15, and 17) whose electrodiagnostic results worsened all showed persistently enlarged nerves [despite a reduction in nerve size in 1 (#15)].
Subjects whose grip strength improved or remained strong were more likely (P=0.04) to have normal or reduced nerve size (12/16) than those whose grip strength weakened (0/3). Sixteen subjects showed either improved grip strength (change in grip strength ≥+39%, n=10) or stable (change in grip between −10% and +22%), relatively strong (strength >266N at baseline) grip strength during follow-up. Of these, nerve size at last follow up remained normal (n=3), normalized (n=6), or became smaller (n=3) in 12, remained enlarged without change in 2 with stable strength (#12 and #23), and enlarged in 2 treatment-dependent subjects (#7 and #22) with improved grip strength (change in grip +134% and +189%). Grip strength weakened during follow-up (change in grip strength between −27% to −51%) in 3 subjects (#2,15, and 17); all had enlarged nerves during the interval when grip weakened. In subjects with enlarged nerves who weakened, nerves could become smaller during periods of adequate treatment. For example, #15 had persistently enlarged nerves and initial worsening in strength off medications. Subsequent steroid treatment yielded a reduction in nerve size of 73% while grip strength improved to baseline (133N to 267N). At last follow-up, the percent change from baseline in grip strength did not correlate (P=0.5) with percent change in nerve size (rs =0.2).
Relatively rapid changes in nerve size were seen in the 2 treatment-naïve subjects with enlarged nerves at baseline. One (#21) showed a 39% reduction in nerve size without significant change in grip strength (209N to 222N) 2 days after treatment initiated with methylprednisolone. Her strength later increased to 423N without continued change in nerve size. The other (#3) showed a 128% reduction in nerve size and increase in strength (187N to 436N) 3 months after treatment with methylprednisolone and IVIG.
Nerves could also decrease in size during remission after discontinuation of medication without change in strength. One (#5) showed a 43% reduction in nerve size without significant change in strength (267N to 254N) over 21 months from baseline measurements 1 year after treatment cessation. Another (#11) showed normalization of nerve size without significant change in strength (454N to 409N) over 2.5 years from baseline measurements 7 months after treatment cessation.
In some chronically treated patients, enlarged nerves normalized years after symptom onset [49 (16–142) months] (Figure 1). The 3 chronically treated subjects with disease duration longer than 16 years had persistently and greatly enlarged nerves and did not achieve remission.
Discussion
These results show that changes in nerve size over time in CIDP vary with treatment response. In CIDP, nerve size is often increased9,15,16,19. Over time, with treatment, nerve size reduced on average. We examined the relation of changes in nerve size to clinical and electrodiagnostic outcome measures, including changes in grip strength, ability to tolerate medication tapering, and persistence of electrodiagnostic features of demyelination. In all, improved outcomes were associated with normal or reduced nerve size. Normal sized nerves or reductions in nerve size were more likely in subjects who had improved or stable, strong grip strength, who tolerated medication tapering, or who had resolution of demyelinating features. In contrast, enlarging nerves or large nerves that fail to decrease in size with treatment suggest a more refractory disease that may fail treatment wean. 7/10 subjects who did not achieve remission and all 3 patients whose strength weakened showed persistently enlarged or enlarging nerves.
There can be acute and chronic resolution of nerve enlargement in CIDP. Rapid improvement occurred in a few patients over a period of days and months after treatment initiation. Other patients showed improvement in nerve size during a period of remission off medications. Some patients on chronic treatment showed normalization of nerve size years after disease onset. Pathologic changes associated with enlarged nerves include increased intraneural blood flow19, intraneural edema, inflammatory changes, onion bulb formation, or accumulation of intraneural proteinaceous material. The specific pathologic changes that accompany the rapidly and chronically reversible nerve enlargement remain to be defined. Prior ultrasound studies in CIDP have recently shown increased intraneural blood flow19 and nerve echogenicity16. Our ultrasound study did not include assessment of intraneural blood flow or nerve echogenicity.
Comparisons of serial ultrasound results can be useful to assess response to treatment in CIDP, as a reduction in nerve size can suggest effective therapy even when nerves remain enlarged. Reduction but not resolution of nerve enlargement was seen in 3 of 13 subjects who achieved clinical remission, in 3 of 16 subjects with improved or stable, strong grip strength, and in 2 of 7 subjects with resolution of demyelinating electrodiagnostic features. In these cases, the interval reduction in nerve size, despite persistent enlargement, supports effective treatment.
Changes in nerve size in CIDP may not parallel precisely changes in grip strength. The degree of change in grip strength did not correlate with degree of change in nerve size. While most (8/10) patients with improved grip strength had normal or reduced nerve size, in 2 treatment-dependent subjects nerves enlarged despite improved grip strength. In these cases, the enlarging nerves might reflect residual disease activity and suggest a need for ongoing treatment. Conversely, reduction in nerve size without clinical change could support adequate treatment. In 1 patient, the rapid nerve size reduction after treatment preceded improvements in strength. In others, nerves normalized or reduced while strength remained stable off medications. The time course of changes in nerve size relative to clinical and electrodiagnostic response following treatment in a population of subjects with CIDP will require further study.
This retrospective study has additional limitations. Subjects were assessed at varying time intervals, sometimes many months apart. Many had been exposed to treatment prior to the initial ultrasound. Prospective studies of treatment-naïve patients with serial follow-up over short, uniform intervals are required to determine the relative timing of changes in nerve size and clinical course following treatment initiation. Some changes in nerve size and function may not have been captured, as electrodiagnostic assessment was not performed with every ultrasound study, and measured outcomes included only grip strength and size of 2 nerves in the arm at 2 locations. Future imaging studies of additional nerves and locations with comparisons to standardized clinical and electrodiagnostic assessments could more completely evaluate how nerve morphology relates to function.
In conclusion, enlarged nerves either decrease or normalize in size in many CIDP patients who improve on medication and who tolerate dose reductions without relapse. In CIDP patients with refractory, active disease, nerves generally enlarge or remain enlarged without significant change over time. Ultrasound at the time of diagnosis can establish baseline nerve size to help guide treatment strategies over time in CIDP.
Supplementary Material
Acknowledgements
The study was supported by the Washington University Neuromuscular Research Fund and the National Institute of Health Neurological Sciences Academic Development Award K12 NS00169009.
Abbreviations
- CIDP
chronic inflammatory demyelinating polyneuropathy
References
- 1.Zaidman CM, Seelig MJ, Baker JC, Mackinnon SE, Pestronk A. Detection of peripheral nerve pathology: comparison of ultrasound and MRI. Neurology. 2013;80:1634–1640. doi: 10.1212/WNL.0b013e3182904f3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padua L, Aprile I, Pazzaglia C, Frasca G, Caliandro P, Tonali P, Martinoli C. Contribution of ultrasound in a neurophysiological lab in diagnosing nerve impairment: A one-year systematic assessment. Clin Neurophysiol. 2007;118:1410–1416. doi: 10.1016/j.clinph.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Padua L, Di Pasquale A, Liotta G, Granata G, Pazzaglia C, Erra C, Briani C, Coraci D, De Franco P, Antonini G, Martinoli C. Ultrasound as a useful tool in the diagnosis and management of traumatic nerve lesions. Clin Neurophysiol. 2013 doi: 10.1016/j.clinph.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 4.Volpe A, Rossato G, Bottanelli M, Marchetta A, Caramaschi P, Bambara LM, Bianconi C, Arcaro G, Grassi W. Ultrasound evaluation of ulnar neuropathy at the elbow: correlation with electrophysiological studies. Rheumatology (Oxford) 2009;48:1098–1101. doi: 10.1093/rheumatology/kep167. [DOI] [PubMed] [Google Scholar]
- 5.Beekman R, Visser LH, Verhagen WI. Ultrasonography in ulnar neuropathy at the elbow: a critical review. Muscle Nerve. 2011;43:627–635. doi: 10.1002/mus.22019. [DOI] [PubMed] [Google Scholar]
- 6.Mhoon JT, Juel VC, Hobson-Webb LD. Median nerve ultrasound as a screening tool in carpal tunnel syndrome: Correlation of cross-sectional area measures with electrodiagnostic abnormality. Muscle & nerve. 2012;46:861–870. doi: 10.1002/mus.23426. [DOI] [PubMed] [Google Scholar]
- 7.Cartwright MS, Hobson-Webb LD, Boon AJ, Alter KE, Hunt CH, Flores VH, Werner RA, Shook SJ, Thomas TD, Primack SJ, Walker FO. Evidence-based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve. 2012;46:287–293. doi: 10.1002/mus.23389. [DOI] [PubMed] [Google Scholar]
- 8.Almeida V, Mariotti P, Veltri S, Erra C, Padua L. Nerve ultrasound follow-up in a child with Guillain-Barre syndrome. Muscle & nerve. 2012;46:270–275. doi: 10.1002/mus.23325. [DOI] [PubMed] [Google Scholar]
- 9.Zaidman CM, Al-Lozi M, Pestronk A. Peripheral nerve size in normals and patients with polyneuropathy: an ultrasound study. Muscle Nerve. 2009;40:960–966. doi: 10.1002/mus.21431. [DOI] [PubMed] [Google Scholar]
- 10.Beekman R, van den Berg LH, Franssen H, Visser LH, van Asseldonk JT, Wokke JH. Ultrasonography shows extensive nerve enlargements in multifocal motor neuropathy. Neurology. 2005;65:305–307. doi: 10.1212/01.wnl.0000169179.67764.30. [DOI] [PubMed] [Google Scholar]
- 11.Martinoli C, Schenone A, Bianchi S, Mandich P, Caponetto C, Abbruzzese M, Derchi LE. Sonography of the median nerve in Charcot-Marie-Tooth disease. AJR American journal of roentgenology. 2002;178:1553–1556. doi: 10.2214/ajr.178.6.1781553. [DOI] [PubMed] [Google Scholar]
- 12.Cartwright MS, Brown ME, Eulitt P, Walker FO, Lawson VH, Caress JB. Diagnostic nerve ultrasound in Charcot-Marie-Tooth disease type 1B. Muscle & nerve. 2009;40:98–102. doi: 10.1002/mus.21292. [DOI] [PubMed] [Google Scholar]
- 13.Granata G, Pazzaglia C, Calandro P, Luigetti M, Martinoli C, Sabatelli M, Padua L. Ultrasound visualization of nerve morphological alteration at the site of conduction block. Muscle & nerve. 2009;40:1068–1070. doi: 10.1002/mus.21449. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka N, Kohriyama T, Ochi K, Nishitani M, Sueda Y, Mimori Y, Nakamura S, Matsumoto M. Detection of cervical nerve root hypertrophy by ultrasonography in chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Sci. 2004;219:15–21. doi: 10.1016/j.jns.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto T, Ochi K, Hosomi N, Takahashi T, Ueno H, Nakamura T, Nagano Y, Maruyama H, Kohriyama T, Matsumoto M. Ultrasonographic nerve enlargement of the median and ulnar nerves and the cervical nerve roots in patients with demyelinating Charcot-Marie-Tooth disease: distinction from patients with chronic inflammatory demyelinating polyneuropathy. J Neurol. 2013 doi: 10.1007/s00415-013-7021-0. [DOI] [PubMed] [Google Scholar]
- 16.Padua L, Granata G, Sabatelli M, Inghilleri M, Lucchetta M, Luigetti M, Coraci D, Martinoli C, Briani C. Heterogeneity of root and nerve ultrasound pattern in CIDP patients. Clin Neurophysiol. 2013 doi: 10.1016/j.clinph.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 17.Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Nerve ultrasound score in distinguishing chronic from acute inflammatory demyelinating polyneuropathy. Clin Neurophysiol. 2013 doi: 10.1016/j.clinph.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Zaidman CM, Harms MB, Pestronk A. Ultrasound of inherited vs. acquired demyelinating polyneuropathies. J Neurol. 2013 doi: 10.1007/s00415-013-7123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goedee HS, Brekelmans GJ, Visser LH. Multifocal enlargement and increased vascularization of peripheral nerves detected by sonography in CIDP: A pilot study. Clin Neurophysiol. 2013 doi: 10.1016/j.clinph.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 20.Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Correlation of nerve ultrasound, electrophysiological, and clinical findings in post Guillain-Barre syndrome. J Peripher Nerv Syst. 2013;18:232–240. doi: 10.1111/jns5.12037. [DOI] [PubMed] [Google Scholar]
- 21.Van den Bergh PY, Pieret F. Electrodiagnostic criteria for acute and chronic inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 2004;29:565–574. doi: 10.1002/mus.20022. [DOI] [PubMed] [Google Scholar]
- 22.Proper performance and interpretation of electrodiagnostic studies. Muscle Nerve. 2006;33:436–439. doi: 10.1002/mus.20493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.