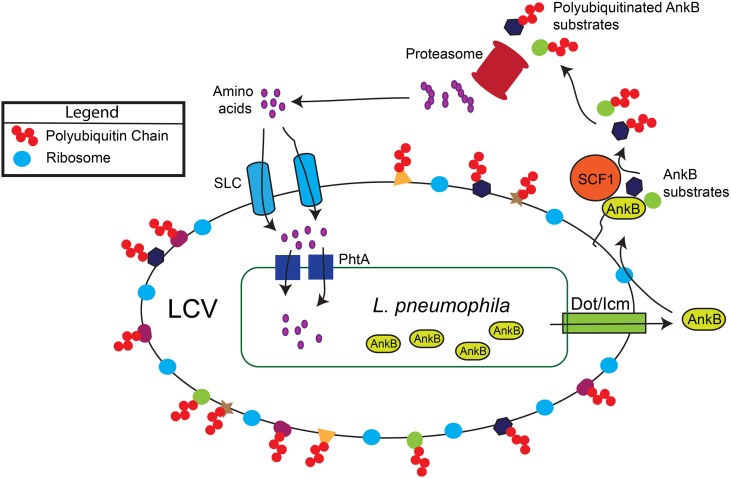
Figure 1.
Generation of a surplus of host amino acids by L. pneumophila and their import into the LCV. The AnkB effector is translocated into macrophages and amoebae by the Dot/Icm type IV secretion system of L. pneumophila. AnkB is immediately farnesylated and anchored into the cytosolic face of the LCV membrane where it interacts with the eukaryotic SCF1 ubiquitin ligase complex. The AnkB effector functions as a platform for the docking of Lys 48-linked polyubiquitinated proteins to the LCV that are subsequently degraded by the proteasome, which generates a surplus of cellular amino acids above the threshold necessary for intra-vacuolar proliferation. The amino acids are likely imported into the LCV through various host SLC amino acid transporters in the LCV membrane, but the identity of the transporters is still to be determined. The amino acids are acquired by L. pneumophila through numerous ABC transporters and amino acid permeases such as the threonine transporter PhtA.