Abstract
The last decade has witnessed significant progress in the field of cancer immunotherapy. This has, in part, been driven by a growing recognition that elements of the innate immune response can be harnessed to induce robust immunity against tumor-associated targets. Nonetheless, as clinically effective immunotherapy for the majority of cancers remains a distant goal, attention has shifted toward multimodality approaches to cancer therapy, sometimes combining novel immunotherapeutics and conventional therapeutics. The traditional view of radiation therapy as immunosuppressive has been challenged, prompting a re-evaluation of its potential as an adjunct to, or even a component of immunotherapy. Radiation therapy may enhance expression of tumor-associated antigens, induce targeting of tumor stroma, diminish regulatory T-cell activity and activate effectors of innate immunity such as dendritic cells through Toll-like receptor (TLR)-dependent mechanisms. Here, we review recent progress in the field of dendritic cell-based immunotherapy, evidence for radiation-induced antitumor immunity and TLR signaling and the results of efforts to rationally integrate radiation into dendritic cell-based immunotherapy strate gies.
INTRODUCTION
The role of the immune system in influencing cancer pathogenesis was controversial for most of the 20th century. Notwithstanding, the apparent susceptibility of immunosuppressed patients to specific cancers and the efficacy of a variety of nonspecific but immunomodulatory agents suggested an important role of immunity in tumorigenesis. In recent years, Schreiber and other investigators made a compelling case for the existence of cancer immunosurveillance (1, 2). Their proposed model of “immunosurveillance and immunoediting” emphasizes the interplay between the immune system and evolving cancers. In this model, early immunogenic tumor cells are eliminated, while less immunogenic tumor cells persist. In later disease, tumors escape immune control by numerous mechanisms including the elaboration of factors such as TGF-β, PGE2, IL-10, gangliosides and indoleamine 2, 3-dioxygenase. It has also become clear that the tumor microenvironment in later stage cancer is enriched with immunosuppressive populations of cells, particularly CD4+CD25+ regulatory T cells (Treg), plasmacytoid dendritic cells (DCs) (3) and immature myeloid cells (4). These elements constitute an important barrier to strategies aimed at the induction of immune responses against tumor-associated antigens. Indeed, most experiences with active immunotherapies have yielded disappointing results. Recent progress in the study of innate immunity has redirected investigators in the field of cancer immunotherapy towards the use of microbial derived adjuvants. This experience has yielded some more promising outcomes and has provided mechanistic insights into the efficacy of some conventional therapies. A growing understanding of the interplay between effectors, antigen-presenting cells and the tumor microenvironment has led immune-based therapies that have a significant impact in some diseases. The role of radiation as an immune activator has increasingly appreciated and an evolving field of investigation focuses on the integration of radiotherapy in immune-based therapies. Here, we review recent progress in the field of dendritic cell-based immunotherapy, evidence for radiation-induced antitumor immunity and Toll-like receptor (TLR) signaling and the results of efforts to rationally integrate radiation into dendritic cell-based immunotherapy strategies.
TLRs IN INNATE AND ADAPTIVE IMMUNITY
Investigations into the developmental biology of fly larvae led to the identification of the Toll gene Drosophila and the transmembrane receptor it encodes (5). The demonstration that loss-of-function mutations in the Toll gene result in susceptibility to fungal infection, but not to gram negative bacterial infection (6) led to the extensive characterization of immune responses in Drosophila. A parallel search for similar features of immunity in mammals followed (7). Eleven TLRs with homology to invertebrate counterparts were subsequently identified in humans. Toll-like receptors recognize molecular patterns common to many pathogens, termed pathogen-associated molecular patterns (8). These include: double-stranded RNA, which is recognized by TLR3 (9); lipopolysaccharide (LPS), which is recognized by TLR4 (10); single-stranded RNA, which is recognized by TLR7/8 (11); and DNA containing the unmethylated CpG motif, which is recognized by TLR9 (12). Additionally, endogenous ligands of TLRs have been identified, including heat-shock proteins (13) and degradation products of endogenous macromolecules (e.g., heparan sulfate) (14).
An extensive investigation into the molecular basis of TLR function has been undertaken in recent years (15). Though considerable overlap exists between implicated pathways, signaling through the various TLRs may initiate discreet downstream molecular events (Fig. 1). Most TLRs act through the myeloid differentiation primary response protein 88 (MyD88) and tumor necrosis factor receptor-associated factor 6 to activate nuclear factor κB (NF-κB) and mitogen-activated protein kinases and thereby induce gene transcription. MyD88-independent signaling through the TIR-domain-containing adapter inducing IFN-β (TRIF) and the TRIF-related adapter molecule after TLR3 and 4 binding, induce the transcription factor interferon regulatory factor-3 (IRF-3).
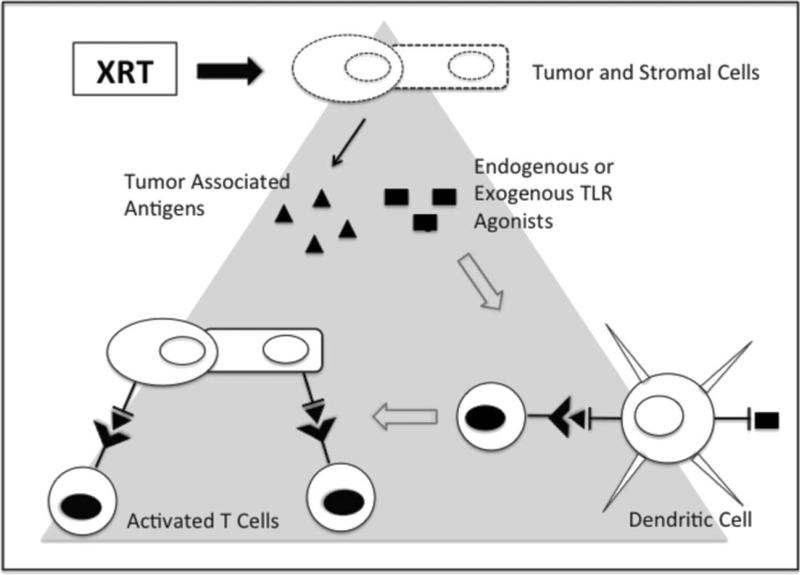
FIG. 1.
Radiation-induced activation of innate immunity through TLR mediated mechanisms. Irradiation (XRT) of tumor cells induces release of tumor-associated antigens (TAA). Radiation-induced release of endogenous TLR agonists or addition of exogenous TLR agonists activate dendritic cells and prime specific T-cell responses against TAA. Activated T-cells target TAA expressing tumor cells as well as stromal cells cross-presenting antigens released from irradiated tumor cells. Dotted lines represent radiation-induced effects on cells.
Importantly, TLR binding results not only in the activation of effectors of innate immunity, but also in the induction of adaptive responses. Ligation of TLRs expressed by antigen presenting cells (APC) may result in the expression of co-stimulatory molecules (e.g., CD80 and CD86) and cytokines (e.g., IL-6 and IL-12) (16). Moreover, TLR-primed dendritic cells induce antigen-specific high avidity CD8+ (17, 18) and type-I polarized CD4+ T-cell responses (19). These findings have provided a foundation for immunotherapeutic strategies targeting tumor-associated antigens for the treatment of malignancies.
TLR AGONISM AS CANCER IMMUNOTHERAPY
Potential therapeutic applications of microbial derived elements have long been recognized. William B. Coley's “toxin” was perhaps the most notable historic example – a preparation of killed Serratia and Streptococci, which he administered to patients with various malignancies. In recent years, Janeway and colleagues provided mechanistic insight into the basis of such classical immunization strategies through the identification of TLRs and pathogen-associated molecular patterns (8). Through the induction of antigen-presenting cell maturation and secretion of proinflammatory chemokines and cytokines, TLRs provide a link between innate and adaptive immunity, which may be exploited to induce robust immune responses against specific antigens. The therapeutic potential of TLR activation in a variety of clinical settings is becoming increasingly evident (20). Established cancer therapeutics, including the Bacillus Calmette–Guerin (BCG) cell-wall skeleton (CWS) and imiquimod, used for the treatment of bladder cancer and basal cell skin cancer, respectively, act on TLRs (21, 22). Moreover, numerous preclinical and clinical trials have utilized TLR agonists as vaccine adjuvants. CpG oligodeoxynucleotides (ODN), which act on TLR9, have been studied most extensively in this setting and appear to enhance vaccine immunogenicity (23, 24). Limitations of such strategies are likewise apparent, as uniform clinical responses remain elusive. While improvements in the design of immunotherapies may result in enhanced efficacy, conventional adjunctive therapies may have a role as well. Immunomodulatory effects of cytotoxic chemotherapy may result in enhanced immunogenicity of TLR-targeted immunotherapies. Conversely, TLR-targeted therapy may enhance subsequent responses to chemotherapy. Such combinations have been explored in a number of preclinical studies, sometimes with promising results (25– 27). Shi et al. demonstrated enhanced chemosensitivity of chronic lymphocytic leukemia cells after tolerizing treatment with the TLR7 agonist, S28690 (27). The addition of a TLR9 agonist was shown to yield improved clinical responses to chemotherapy in patients with non-small cell lung cancer (28).
EFFECTS OF RADIATION EXPOSURE ON DENDRITIC CELLS
Radiation therapy has traditionally been viewed as immunosuppressive (29–31). Lymphocyte radiosensitivity is well established and remains the dominant explanation for this effect. However, substantial evidence suggests more varied effects of radiation exposure on the immune system, prompting the re-characterization of radiation as “immunomodulatory” rather than immunosuppressive (32). Increased expression of proinflammatory cytokines, including TNF-α and IL-1β after irradiation has been reported (33– 36). These observations suggest a potential role for radiation in signaling “danger” and in the activation of antigen-presenting cells (32). However, studies aimed at determining the effects of radiation exposure on antigen-presenting cell phenotype, cytokine expression and function have led to somewhat divergent conclusions. While alterations in DC phenotype have been demonstrated infrequently (37), a number of investigators have noted changes in the cytokine secretory profile and function of DCs after irradiation. For example, Shigematsu et al. reported enhanced expression of IL-2, IL-12 and IFN-γ by irradiated DCs (38). This expression profile correlated with greater T-cell proliferation after co-culture with irradiated DCs compared to nonirradiated DCs. In contrast, Merrick et al. reported decreased IL-12 production and impaired naive T-cell priming by irradiated DCs compared to nonirradiated DCs (39).
The effect of radiation on DC antigen presentation also remains controversial with a number of studies suggesting significant modulation of, or no effect on, T-cell stimulatory capacity. Conversely, Liao et al. reported impaired T-cell priming against endogenously processed antigen and enhanced priming against exogenous peptide (40), suggesting a more complex interplay between radiation and DC function. A number of factors may explain why the consistent characterization of immune responses to radiation remains elusive include differences in radiosensitivity or reactivity of different cell and tissue types and dose-dependent effects. The latter have been explored in a number of studies, some of which suggest that lower doses of radiation have a greater potential to enhance immune responses (38, 41–43). Optimal dosing regimens and the complex interplay between radiation dose and delivery with target tissue-associated factors and host immunity have yet to be consistently defined (44).
RADIATION-INDUCED TLR SIGNALING IN DENDRITIC CELLS
Investigations into the function of TLRs have provided mechanistic insight into the actions of several established cancer therapies. Recent evidence suggests that TLR-dependent mechanisms contribute to the therapeutic effects of radiation as well. It has been widely hypothesized that tumor irradiation activates effectors of innate immunity through the induction of tumor cell apoptosis and the release of endogenous TLR agonists (45, 46). The observation that such ubiquitous factors as heat-shock proteins and uric acid can act through TLRs and induce DC maturation (47) supports this mechanism as does the demonstration that immature DCs, when administered into irradiated tumors, induce antitumor immunity (48). More recently, the high-mobility-group box 1 alarmin protein, released by dying tumor cells, was shown to act on TLR4 expressed by DCs. Moreover, binding of TLR4 was demonstrated to increase the efficiency of tumor antigen processing and presentation (49). TLR-dependent mechanisms may play a role in systemic therapies as well. Whole-body irradiation was shown to increase bacterial translocation and circulating levels of the TLR4 agonist lipopolysaccharide. This phenomenon was associated with enhanced antitumor immunity in an adoptive transfer model (50).
When considered together, such evidence provides a strong rationale for the use of radiation therapy as an immune intervention; a paradigm shift from the traditional view of radiation therapy as a cytotoxic or cytoreductive therapy. The capacity of radiation to elicit the expression of TLR ligands systemically after gastrointestinal tract irradiation or locally after tumor irradiation, may prove valuable in conjunction with other immunotherapies. Pursuit of such approaches, however, must be tempered by the recognition that TLR agonism may have other unanticipated consequences. Indeed, data is emerging that activation of particular pathways (e.g., after TLR4 or TLR9 agonism) may contribute to radioresistance (51).
PROVIDING ANTIGEN FOR DENDRITIC CELL PRESENTATION
Induction Of antitumor immunity by exposure to radiation may result from enhanced tumor antigen recognition. Radiation induces tumor cell apoptosis or necrosis secondary to vascular injury (52). Subsequent phagocytosis of apoptotic bodies by DCs and initiation of antitumor T-cell responses through cross presentation may ensue if DC maturation signals are concomitantly present. A number of studies have suggested that necrosis, but not apoptosis, is associated with DC maturation signals (53, 54). However, more recent evidence suggests that some apoptotic pathways do induce DC maturation and antitumor immunity (55). Sublethal irradiation of tumors may also result in enhanced expression of surface molecules recognized or targeted by immune effectors, as is suggested by studies demonstrating increased expression of the MHC class I antigen, H-2D, by melanoma cells (56) and tumor-associated antigens including carcinoembryonic antigen (CEA) by gastric adenocarcinoma cells (57). Increased expression of the death receptor Fas after irradiation has also been demonstrated in a transgenic CEA-expressing tumor model and associated with greater susceptibility to cytotoxic T lymphocyte (CTL)-mediated tumor cell lysis (58).
RADIATION EFFECTS ON THE MICROENVIRONMENT
As noted above, the tumor microenvironment is enriched with a variety of immunosuppressive elements. Radiation exposure may alter the microenvironment to allow for more robust antitumor immunity. Suppressor populations of T cells may be more radiosensitive than their effector counterparts and, conversely, tumor-specific effector T cells may be relatively radioresistant (59, 60). Enhanced functionality of adoptively transferred T cells after radiation-induced lymphodepletion has also been linked to increased availability of homeostatic cytokines (61). A number of studies have measured the effects of low-dose total-body irradiation on the relative size of T-cell subpopulations and expression of cytokines associated with T-cell activation. In one report, low-dose irradiation after transplantation of a hepatoma cell line in a rat model resulted in an increased proportion of CD8+ splenocytes, increased numbers of tumor-infiltrating lymphocytes, increased TNF-α and IFN-γ expression and decreased TGF-β expression. This response correlated with a reduction in metastases (41). Another study demonstrated an increased CD4+/CD8+ T-cell ratio and decreased expression of TGF-β and VEGF after low-dose total-body irradiation, which correlated with delayed tumor growth in mice transplanted with the Lewis lung carcinoma cell line (62).
Radiation may also induce immune-mediated targeting of tumor stroma. Antigen released after tumor irradiation may be presented by stromal cells for subsequent destruction by CTLs. This mechanism was recently demonstrated by Zhang et al. in experiments utilizing immunodeficient mice xenotransplanted with tumor. Only the combination of radiation and adoptive transfer of CTLs resulted in tumor regression, and correlated with increased expression of tumor-specific peptide-MHC complexes as delineated using a tumor antigen/MHC complex-specific TCR tetramer (63). Direct effects of radiation on the stroma may play a role in enhancing immune-mediated tumor regression as well. Induced modulation of the expression of adhesion molecules, such as accumulation of P-selectin in the lumen of tumor vasculature, may enhance infiltration of immune effectors into the tumor stroma (64, 65).
A dynamic interplay between irradiated tumor cells, stromal elements and immune mediators has also been implicated in tumor recurrence after radiation therapy. Indeed, stromal cellular elements including endothelial and myeloid cells have been implicated in tumor cell resistance and tumor recurrence after irradiation (66–68). Recruitment of myeloid precursors into the tumor microenvironment early after irradiation appears to presage recurrence (69, 70). Additionally, Huang et al. reported that dying tumor cells use the apoptotic process to generate potent growth-stimulating signals including elaboration of caspase-3 to stimulate the repopulation of tumors undergoing radiotherapy (71). These data highlight the potential pitfalls of reductive models that cast radiation as uniformly tumoricidal or tumorigenic and the need for a more nuanced understanding of the effects of radiation.
COMBINING DENDRITIC CELL THERAPY AND RADIATION EXPOSURE
Building upon the hypothesis that radiation exposure can enhance antitumor immunity, investigators have begun to combine radiation therapy with immunotherapies (72). Generally, such efforts employ radiation exposure to induce tumor cell apoptosis or necrosis with resultant antigen release for subsequent presentation by DCs. Several investigators have studied combinations of intratumoral or peritumoral DC administration (73) or administration of Flt-3L (74–76) combined with radiation exposure and have achieved promising results. For example, administration of a recombinant viral vaccine expressing tumor-associated antigen(s) and costimulatory molecules in combination with tumor irradiation was explored by Chakraborty and colleagues. Enhanced immune recognition of antigen-expressing tumor cells was associated with radiation-induced upregulation of Fas on tumor cells (77). Other investigators have explored combinations of cytokine therapy and irradiation, using cytokines IL-3 (78), IL-12 (79, 80) and TNF-α (81). Local radiation therapy in combination with CTLA-4 blockade is an additional novel approach for overcoming mechanisms of tumor tolerance. This combination was recently demonstrated to induce antitumor CD8+ T cells in a poorly immunogenic murine adenocarcinoma model, whereas CTLA-4 blockade alone did not (82). Results of a clinical trial of CTLA-4 blockade and concurrent radiotherapy (RADVAX) are eagerly awaited.
LATE EFFECTS OF RADIATION THERAPY AND VACCINATION
We have conducted clinical trials of an LPS (TLR4 agonist)-primed, HER-2/neu peptide-pulsed DC vaccine for patients with HER-2/neu overexpressing ductal carcinoma in situ (DCIS) of the breast (83). The vaccines consisted of HER2 peptide-pulsed autologous DC activated with IFN-γ and clinical-grade TLR agonist LPS, resulting in high-level IL-12 and Th1 chemokine secretion (DC1). The results suggested consistent immune sensitization and frequent clinical response. Vaccination induced infiltration of CD4+ helper T cells, CD8+ CTLs and CD20+ B cells around DCIS lesions. Marked reduction of HER2 expression in lesions was frequently observed. In HLA-A2.1+ subjects, post-vaccination CD8+ T cells routinely recognized HER2-positive breast cancer cell lines (but not HER2-negative lines) and specifically secreted IFN-γ in vitro. Moreover, the immunity generated by this vaccination regimen proved to be long-lived. All evaluated subjects had long-term CD4 T-cell immunity to vaccine peptides demonstrated by ELISPOT (83).
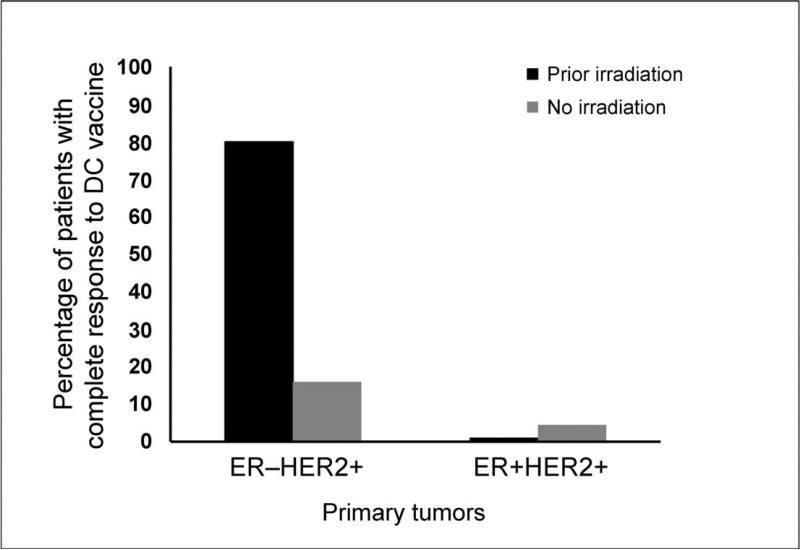
We recently compared clinical and immune responses post-vaccination in estrogen-dependent and estrogen-independent patients (ER+ and ER−, respectively). Interestingly, complete tumor regression was significantly more common in patients with ER-independent DCIS (40% of patients) compared to ER-dependent DCIS (4% of patients). Moreover, those patients who had received radiotherapy to the ipsilateral breast for a previous breast cancer event derived particular benefit compared to patients with ER-dependent disease and those who had received prior irradiation to the contralateral breast (Fig. 2) (84). These results suggest that radiation may have a sustained local effect that enhances subsequent vaccine efficacy. Observations such as these highlight limitations in our current understanding of the interactions between radiation therapy and DC-based immunotherapy. Determining the durability of radiation-induced changes in the tumor microenvironment and the optimal timing and sequencing of combined therapies will be important areas for further investigation.
FIG. 2.
Prior ipsilateral breast irradiation increases complete response rate to HER2-targeted dendritic cell (DC) vaccine. Patients with HER2+ breast ductal carcinoma in situ (DCIS) were treated with HER2-pulsed type 1-polarized DC vaccine prior to surgery. Residual disease at the time of surgery was assessed, and percentage of patients displaying complete response (CR) is shown. ER-independent patients who had prior ipsilateral breast irradiation showed particularly marked tumor regression compared with ER-dependent patients or those who had received no prior irradiation or prior irradiation to the contralateral breast only.
COMBINING RADIATION THERAPY WITH TOLL-LIKE RECEPTOR AGONISTS
Few investigators have directly studied the combination of radiation therapy and TLR-targeted therapies. Mason et al. demonstrated a markedly enhanced tumor response to radiation therapy after peritumoral and intratumoral injections of the TLR agonist, CpG, in a murine fibrosarcoma model (85, 86). Increased radiosensitivity of lung cancer cell lines after treatment with CpG has also been reported (87). In light of the recently elucidated role of TLRs in radiation-induced responses, this effect may reflect synergy at the level of TLR signaling (Fig. 1). Further investigations are required to determine the applicability of such approaches, but the potential implications of these findings are broad. TLR-targeted therapy may sensitize a wide range of tumor types to radiation therapy and limit the radiation dose required to achieve therapeutic effect. Related approaches may become more prevalent as new TLR-targeted therapies emerge.
FUTURE DIRECTIONS
The armamentarium of immunotherapeutics is expanding at a rapid rate. DC-based immunotherapy in particular shows promise, as DC vaccines are increasingly being administered in a neoadjuvant setting, allowing for the identification of more objective surrogates for response. This approach may also facilitate the study of combinations of immunotherapies and conventional therapeutics, such as chemotherapy and radiation therapy. Radiation therapy may enhance the antigenicity of tumors and promote stromal targeting, and perhaps more importantly, may activate effector DCs through TLR-dependent mechanisms. The addition of radiotherapy could enhance the efficacy of DC-based therapies, however, deficiencies in our current understanding of the power and limitations of such approaches preclude rational treatment design. Important areas of uncertainty include the optimal dosing, timing and sequencing of therapies. As we learn more about the immune-mediated mechanisms initiated by radiotherapy we will be able to utilize this modality more rationally.
ACKNOWLEDGMENTS
Research from our laboratory cited in this manuscript was supported by NIH R01 CA096997 and by the Pennies in Actiont fund (www.penniesinaction.org).
REFERENCES
- 1.Schreiber RD. Cancer vaccines 2004 opening address: the molecular and cellular basis of cancer immunosurveillance and immunoediting. Cancer Immun. 2005;5(Suppl 1):1. [PubMed] [Google Scholar]
- 2.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–45. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto C, Hudson KL, Anderson KV. The toll gene of drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988;52:269–79. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- 6.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/toll/cactus controls the potent antifungal response in drosophila adults. Cell. 1996;86:973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 7.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 8.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annual Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature. 2001;413:732–8. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 10.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in TLR4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 11.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 12.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 13.Wallin RP, Lundqvist A, More SH, von Bonin A, Kiessling R, Ljunggren HG. Heat-shock proteins as activators of the innate immune system. Trends Immunol. 2002;23:130–5. doi: 10.1016/s1471-4906(01)02168-8. [DOI] [PubMed] [Google Scholar]
- 14.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by toll-like receptor 4. J Immunol. 2002;168:5233–9. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 15.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 16.Macagno A, Napolitani G, Lanzavecchia A, Sallusto F. Duration, combination and timing: the signal integration model of dendritic cell activation. Trends Immunol. 2007;28:227–33. doi: 10.1016/j.it.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Xu S, Koski GK, Faries M, Bedrosian I, Mick R, Maeurer M, et al. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171:2251–61. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 18.Xu S, Koldovsky U, Xu M, Wang D, Fitzpatrick E, Son G, et al. High-avidity antitumor T-cell generation by toll receptor 8-primed, myeloid- derived dendritic cells is mediated by IL-12 production. Surgery. 2006;140:170–8. doi: 10.1016/j.surg.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Wesa A, Kalinski P, Kirkwood JM, Tatsumi T, Storkus WJ. Polarized type-1 dendritic cells (DC1) producing high levels of IL-12 family members rescue patient TH1-type antimelanoma CD4+ T cell responses in vitro. J Immunother. 2007;30:75–82. doi: 10.1097/01.cji.0000211316.15278.6e. [DOI] [PubMed] [Google Scholar]
- 20.van Duin D, Medzhitov R, Shaw AC. Triggering TLR signaling in vaccination. Trends Immunol. 2006;27:49–55. doi: 10.1016/j.it.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji S, Matsumoto M, Takeuchi O, Akira S, Azuma I, Hayashi A, et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: involvement of toll-like receptors. Infect Immun. 2000;68:6883–90. doi: 10.1128/iai.68.12.6883-6890.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ambach A, Bonnekoh B, Nguyen M, Schon MP, Gollnick H. Imiquimod, a toll-like receptor-7 agonist, induces perforin in cytotoxic T lymphocytes in vitro. Mol Immunol. 2004;40:1307–14. doi: 10.1016/j.molimm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Liu HM, Newbrough SE, Bhatia SK, Dahle CE, Krieg AM, Weiner GJ. Immunostimulatory CpG oligodeoxynucleotides enhance the immune response to vaccine strategies involving granulocyte-macrophage colony-stimulating factor. Blood. 1998;92:3730–6. [PubMed] [Google Scholar]
- 24.Kochling J, Konig-Merediz SA, Stripecke R, Buchwald D, Korte A, Von Einsiedel HG, et al. Protection of mice against Philadelphia chromosome-positive acute lymphoblastic leukemia by cell-based vaccination using nonviral, minimalistic expression vectors and immunomodulatory oligonucleotides. Clin Cancer Res. 2003;9:3142–9. [PubMed] [Google Scholar]
- 25.Lake RA, Robinson BW. Immunotherapy and chemotherapy–a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 26.Bourquin C, Schreiber S, Beck S, Hartmann G, Endres S. Immunotherapy with dendritic cells and CpG oligonucleotides can be combined with chemotherapy without loss of efficacy in a mouse model of colon cancer. Int J Cancer. 2006;118:2790–5. doi: 10.1002/ijc.21681. [DOI] [PubMed] [Google Scholar]
- 27.Shi Y, White D, He L, Miller RL, Spaner DE. Toll-like receptor-7 tolerizes malignant B cells and enhances killing by cytotoxic agents. Cancer Res. 2007;67:1823–31. doi: 10.1158/0008-5472.CAN-06-2381. [DOI] [PubMed] [Google Scholar]
- 28.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Invest. 2007;117:1184–94. doi: 10.1172/JCI31414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole S. Long-term effects of local ionizing radiation treatment on Langerhans cells in mouse footpad epidermis. J Invest Dermatol. 1986;87:608–12. doi: 10.1111/1523-1747.ep12455853. [DOI] [PubMed] [Google Scholar]
- 30.James RF, Lake SP, Chamberlain J, Thirdborough S, Bassett PD, Mistry N, et al. Gamma irradiation of isolated rat islets pretransplantation produces indefinite allograft survival in cyclosporine-treated recipients. Transplantation. 1989;47:929–33. doi: 10.1097/00007890-198906000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Wasserman J, Blomgren H, Rotstein S, Petrini B, Hammarstrom S. Immunosuppression in irradiated breast cancer patients: in vitro effect of cyclooxygenase inhibitors. Bull N Y Acad Med. 1989;65:36–44. [PMC free article] [PubMed] [Google Scholar]
- 32.McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 33.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989;86:10104–7. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishihara H, Tanaka I, Nemoto K, Tsuneoka K, Cheeramakara C, Yoshida K, et al. Immediate-early, transient induction of the interleukin-1 beta gene in mouse spleen macrophages by ionizing radiation. J Radiat Res. 1995;36:112–24. doi: 10.1269/jrr.36.112. [DOI] [PubMed] [Google Scholar]
- 35.Nemoto K, Ishihara H, Tanaka I, Suzuki G, Tsuneoka K, Yoshida K, et al. Expression of IL-1 beta mRNA in mice after whole body X-irradiation. J Radiat Res. 1995;36:125–33. doi: 10.1269/jrr.36.125. [DOI] [PubMed] [Google Scholar]
- 36.Hong JH, Chiang CS, Tsao CY, Lin PY, McBride WH, Wu CJ. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421–7. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 37.Cao MD, Chen ZD, Xing Y. Gamma irradiation of human dendritic cells influences proliferation and cytokine profile of T cells in autologous mixed lymphocyte reaction. Cell Biol Int. 2004;28:223–8. doi: 10.1016/j.cellbi.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 38.Shigematsu A, Adachi Y, Koike-Kiriyama N, Suzuki Y, Iwasaki M, Koike Y, et al. Effects of low-dose irradiation on enhancement of immunity by dendritic cells. J Radiat Res. 2007;48:51–5. doi: 10.1269/jrr.06048. [DOI] [PubMed] [Google Scholar]
- 39.Merrick A, Errington F, Milward K, O'Donnell D, Harrington K, Bateman A, et al. Immunosuppressive effects of radiation on human dendritic cells: reduced IL-12 production on activation and impairment of naive T-cell priming. Br J Cancer. 2005;92:1450–8. doi: 10.1038/sj.bjc.6602518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liao YP, Wang CC, Butterfield LH, Economou JS, Ribas A, Meng WS, et al. Ionizing radiation affects human MART-1 melanoma antigen processing and presentation by dendritic cells. J Immuno. 2004;173:2462–9. doi: 10.4049/jimmunol.173.4.2462. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto S, Shirato H, Hosokawa M, Nishioka T, Kuramitsu Y, Matushita K, et al. The suppression of metastases and the change in host immune response after low-dose total-body irradiation in tumor-bearing rats. Radiat Res. 1999;151:717–24. [PubMed] [Google Scholar]
- 42.Cao ZA, Daniel D, Hanahan D. Sub-lethal radiation enhances anti-tumor immunotherapy in a transgenic mouse model of pancreatic cancer. BMC Cancer. 2002;2:11. doi: 10.1186/1471-2407-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ina Y, Sakai K. Activation of immunological network by chronic low-dose-rate irradiation in wild-type mouse strains: analysis of immune cell populations and surface molecules. Int J Radiat Biol. 2005;81:721–9. doi: 10.1080/09553000500519808. [DOI] [PubMed] [Google Scholar]
- 44.Demaria S, Formenti SC. Radiation as an immunological adjuvant: current evidence on dose and fractionation. Front Oncol. 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burnette B, Weichselbaum RR. Radiation as an immune modulator. Semin Radiat Oncol. 2013;23:273–80. doi: 10.1016/j.semradonc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 46.El-Saghire H, Michaux A, Thierens H, Baatout S. Low doses of ionizing radiation induce immune-stimulatory responses in isolated human primary monocytes. Int J Mol Med. 2013;32:1407–14. doi: 10.3892/ijmm.2013.1514. [DOI] [PubMed] [Google Scholar]
- 47.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–55. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 48.Kim KW, Kim SH, Shin JG, Kim GS, Son YO, Park SW, et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int J Cancer. 2004;109:685–90. doi: 10.1002/ijc.20036. [DOI] [PubMed] [Google Scholar]
- 49.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 50.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. The J Clin Invest. 2007;117:2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu C, Zhang C, Mitchel RE, Cui J, Lin J, Yang Y, et al. A critical role of toll-like receptor 4 (TLR4) and its’ in vivo ligands in basal radio-resistance. Cell Death Dis. 2013;4:e649. doi: 10.1038/cddis.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acker JC, Marks LB, Spencer DP, Yang W, Avery MA, Dodge RK, et al. Serial in vivo observations of cerebral vasculature after treatment with a large single fraction of radiation. Radiat Res. 1998;149:350–9. [PubMed] [Google Scholar]
- 53.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 54.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer. 2003;103:205–11. doi: 10.1002/ijc.10777. [DOI] [PubMed] [Google Scholar]
- 56.Hauser SH, Calorini L, Wazer DE, Gattoni-Celli S. Radiation-enhanced expression of major histocompatibility complex class I antigen H-2Db in B16 melanoma cells. Cancer Res. 1993;53:1952–5. [PubMed] [Google Scholar]
- 57.Hareyama M, Imai K, Kubo K, Takahashi H, Koshiba H, Hinoda Y, et al. Effect of radiation on the expression of carcinoembryonic antigen of human gastric adenocarcinoma cells. Cancer. 1991;67:2269–74. doi: 10.1002/1097-0142(19910501)67:9<2269::aid-cncr2820670910>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 58.Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–47. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 59.Dunn PL, North RJ. Selective radiation resistance of immunologically induced T cells as the basis for irradiation-induced T-cell-mediated regression of immunogenic tumor. J Leukoc Biology. 1991;49:388–96. doi: 10.1002/jlb.49.4.388. [DOI] [PubMed] [Google Scholar]
- 60.North RJ. Radiation-induced, immunologically mediated regression of an established tumor as an example of successful therapeutic immunomanipulation. Preferential elimination of suppressor T cells allows sustained production of effector T cells. J Exp Med. 1986;164:1652–66. doi: 10.1084/jem.164.5.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wrzesinski C, Paulos CM, Gattinoni L, Palmer DC, Kaiser A, Yu Z, et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Miller GM, Kim DW, Andres ML, Green LM, Gridley DS. Changes in the activation and reconstitution of lymphocytes resulting from total-body irradiation correlate with slowed tumor growth. Oncology. 2003;65:229–41. doi: 10.1159/000074476. [DOI] [PubMed] [Google Scholar]
- 63.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hallahan DE, Staba-Hogan MJ, Virudachalam S, Kolchinsky A. X-ray-induced P-selectin localization to the lumen of tumor blood vessels. Cancer Res. 1998;58:5216–20. [PubMed] [Google Scholar]
- 65.Hallahan DE, Virudachalam S. Accumulation of P-selectin in the lumen of irradiated blood vessels. Radiat Res. 1999;152:6–13. [PubMed] [Google Scholar]
- 66.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci U S A. 2010;107:8363–8. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 68.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kozin SV, Duda DG, Munn LL, Jain RK. Neovascularization after irradiation: what is the source of newly formed vessels in recurring tumors? J Natl Cancer Inst. 2012;104:899–905. doi: 10.1093/jnci/djs239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kozin SV, Kamoun WS, Huang Y, Dawson MR, Jain RK, Duda DG. Recruitment of myeloid but not endothelial precursor cells facilitates tumor regrowth after local irradiation. Cancer Res. 2010;70:5679–85. doi: 10.1158/0008-5472.CAN-09-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat Med. 2011;17:860–6. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63:655–66. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nikitina EY, Gabrilovich DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94:825–33. doi: 10.1002/1097-0215(20011215)94:6<825::aid-ijc1545>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 74.Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–32. [PubMed] [Google Scholar]
- 75.Chakravarty PK, Guha C, Alfieri A, Beri V, Niazova Z, Deb NJ, et al. Flt3L therapy following localized tumor irradiation generates long-term protective immune response in metastatic lung cancer: its implication in designing a vaccination strategy. Oncology. 2006;70:245–54. doi: 10.1159/000096288. [DOI] [PubMed] [Google Scholar]
- 76.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 77.Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res. 2004;64:4328–37. doi: 10.1158/0008-5472.CAN-04-0073. [DOI] [PubMed] [Google Scholar]
- 78.Chiang CS, Hong JH, Wu YC, McBride WH, Dougherty GJ. Combining radiation therapy with interleukin-3 gene immunotherapy. Cancer Gene Ther. 2000;7:1172–8. doi: 10.1038/sj.cgt.7700217. [DOI] [PubMed] [Google Scholar]
- 79.Seetharam S, Staba MJ, Schumm LP, Schreiber K, Schreiber H, Kufe DW, et al. Enhanced eradication of local and distant tumors by genetically produced interleukin-12 and radiation. Int J Oncol. 1999;15:769–73. doi: 10.3892/ijo.15.4.769. [DOI] [PubMed] [Google Scholar]
- 80.Lohr F, Hu K, Haroon Z, Samulski TV, Huang Q, Beaty J, et al. Combination treatment of murine tumors by adenovirus-mediated local B7/IL12 immunotherapy and radiotherapy. Mol Ther. 2000;2:195–203. doi: 10.1006/mthe.2000.0114. [DOI] [PubMed] [Google Scholar]
- 81.Weichselbaum RR, Hallahan DE, Beckett MA, Mauceri HJ, Lee H, Sukhatme VP, et al. Gene therapy targeted by radiation preferentially radiosensitizes tumor cells. Cancer Res. 1994;54:4266–9. [PubMed] [Google Scholar]
- 82.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 83.Czerniecki BJ, Koski GK, Koldovsky U, Xu S, Cohen PA, Mick R, et al. Targeting HER-2/neu in early breast cancer development using dendritic cells with staged interleukin-12 burst secretion. Cancer Res. 2007;67:1842–52. doi: 10.1158/0008-5472.CAN-06-4038. [DOI] [PubMed] [Google Scholar]
- 84.Fracol M, Xu S, Mick R, Fitzpatrick E, Nisenbaum H, Roses R, et al. Response to HER-2 pulsed DC1 vaccines is predicted by both HER-2 and estrogen receptor expression in DCIS. Ann Surg Oncol. 2013;20:3233–9. doi: 10.1245/s10434-013-3119-y. [DOI] [PubMed] [Google Scholar]
- 85.Mason KA, Ariga H, Neal R, Valdecanas D, Hunter N, Krieg AM, et al. Targeting toll-like receptor 9 with CpG oligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clin Cancer Res. 2005;11:361–9. [PubMed] [Google Scholar]
- 86.Milas L, Mason KA, Ariga H, Hunter N, Neal R, Valdecanas D, et al. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64:5074–7. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 87.Yan L, Xu G, Qiao T, Chen W, Yuan S, Li X. CpG-ODN 7909 increases radiation sensitivity of radiation-resistant human lung adenocarcinoma cell line by overexpression of toll-like receptor 9. Cancer Biother Radiopharm. 2013;28:559–64. doi: 10.1089/cbr.2012.1450. [DOI] [PubMed] [Google Scholar]