| Drug name (generic) | Ruxolitinib (INCB018424) |
| Phase | Currently in Phase III |
| Indication | Treatment of relapsed/refractory leukemia |
| Pharmacology description/mechanism of action | Selective inhibitor of JAK1 and JAK2 |
| Route of administration | Oral |
| Chemical structure | C17H21N6O4P* |
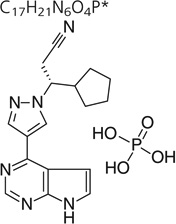
|
|
| Pivotal trial(s) | Patients with either primary or post-ET and post-PV myelofibrosis (NCT:00952289) |
| Patients with polycythemia that are hydroxyurea resistant, refractory, or intolerant (NCT:01243944) |
ET: Essential thrombocythemia; PV: Polycythemia vera.
