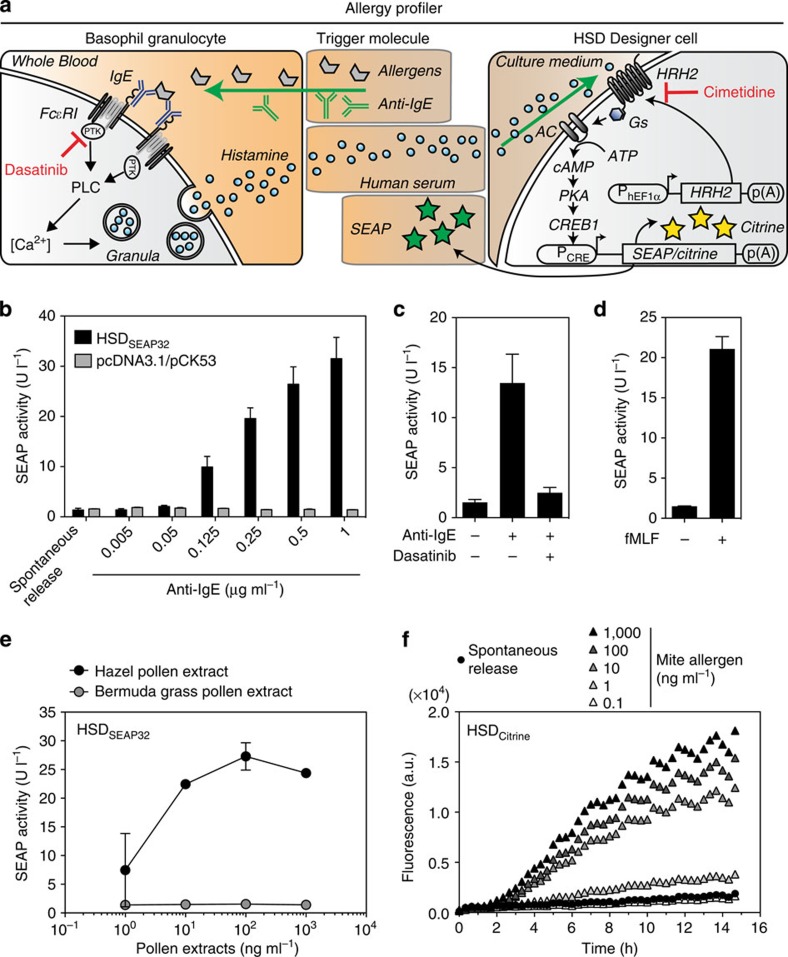
Figure 2. Human basophil-derived histamine triggers reporter gene expression in HSD-engineered designer cells.
(a) Illustration of the cell-dependent allergy profiler. Allergens added to the blood sample bind to IgE, which crosslink membrane-bound high-affinity IgE receptors (FcεRI) of basophil granulocytes. FcεRI activation induces a protein tyrosine kinase (PTK)- and phospholipase C (PLC)-dependent signal transduction cascade, resulting in an intracellular calcium (Ca2+) surge and a subsequent release of histamine. Histamine binds to HRH2 on the HSD designer cells and triggers dose-dependent reporter gene expression (see Fig. 1a). (b) Adjustable anti-IgE antibody-mediated basophil degranulation triggered SEAP secretion in HSDSEAP32 cells, but not in pcDNA3.1/pCK53-co-transfected control cells lacking the HRH2 sensory module. (c) Inhibition of protein tyrosine kinases by dasatinib (1 μM) in basophils inhibited anti-IgE antibody-mediated degranulation (0.25 μg ml−1 anti-IgE) and, therefore, HSDSEAP32-mediated reporter gene expression. (d) Detection of histamine derived from the IgE-independent fMLF-mediated induction of basophil degranulation (1 μM fMLF) by HSDSEAP32 cells. (e) HSDSEAP32 cells specifically converted hazel pollen-triggered histamine from an allergic donor to a reporter gene response, whereas Bermuda grass pollens repressed the designer cell responses. (f) Real-time detection of histamine derived from mite-induced basophil degranulation by HSDCitrine-engineered cells. The data represent the means±s.d. (n≥3 experiments).