Abstract
Pancreas and islet transplant recipients are monitored using various metabolic and imaging methods. The inaccessibility of the transplanted whole pancreas and of the isolated islets poses specific problems (eg, all assessment techniques are indirect). Although successful pancreas transplantation typically restores normal glucose homeostasis, islet transplantation into the liver does not completely normalize islet hormone secretion and glucose metabolism. Development of better testing strategies, such as direct islet imaging, will significantly advance the field.
Introduction
Islet function tests comprise a wide range of metabolic and imaging studies, all being hampered by the fact that the cells of interest are tucked away in difficult to reach anatomic locations. However, progress has been made in the transplant procedures as well as in the technical aspects of the assessment strategies described in this article.
Brief History of Pancreas and Islet Transplantation
From December 1966 to December 2004, more than 23,000 pancreas transplants were reported to the international pancreas transplant registry, including more than 17,000 from the United States and almost 6000 from other countries [1]. Sutherland et al. [2] divided this time period into five eras, beginning with a slow start (14 pancreas transplants between 1966 and 1973), then accelerating with the introduction of new surgical techniques and new immunosuppressive agents (eg, era three began in 1994 with the arrival of FK506 [tacrolimus] and era four began 4 years later with the addition of daclizumab and thymoglobulin) [2]. Besides the typical patients with autoimmune-mediated type 1 diabetes mellitus (T1DM) who undergo this procedure, the percentage of recipients labeled as having type 2 diabetes mellitus (T2DM) has continuously increased, accounting for 7.7% of those receiving a simultaneous pancreas kidney transplant in 2002 and 2003 [1].
The American Diabetes Association (ADA) supports the procedure for patients with diabetes who have had, or need, a kidney transplant. In the absence of kidney failure, pancreas transplantation may be considered for patients with diabetes and severe and frequent metabolic instability (ie, recurrent hypoglycemia and/or ketoacidosis) [3]. An ongoing controversy exists with regard to the risk-benefit ratio of pancreas transplantation. Although outcome studies have documented improved glycemia control and quality of life in most pancreas transplant recipients [4], data for both positive and negative impact on life expectancy have been published [1,5]. Even though diabetes-associated complications (eg, nephropathy) should revert or at least not progress with post-transplant normalized glycemia control, this has not been shown conclusively. The question is particularly acute in solitary pancreas recipients [1,5], where immunosuppressive agent-induced nephrotoxicity may trump the salutary effects of improved glycemia. Although introducing steroid-sparing immunosuppressive regimens has improved islet function in the short term, agents such as rapamycin (sirolimus) and FK506 (tacrolimus) are associated with nephropathy, hyperlipidemia, and anemia, all increasing cardiovascular risk in the long term [6–8].
Islet transplantation was seen as a promising alternative to pancreas transplantation because procedure-associated risks are decreased, and transplanting tissue (ie, the exocrine pancreas) irrelevant for diabetes treatment can be minimized, thus eliminating postoperative complications caused by nonislet tissue. Even though enthusiasm for clinical islet transplantation began in the early 1970s, its application was significantly limited, largely because islet preparations were of poor quality and low yield, and similar to the pancreas transplant field, suffered from ineffective immunosuppression leading to early rejection. In the late 1970s, various groups, including Najarian et al. [9] and Largiader et al. [10], described their experience with intraportal and intrasplenic human islet allotransplants in patients with nonautoimmune diabetes. One patient's outcome was deemed successful for at least a 10-month follow-up period. In 1990, Scharp et al. [11] reported similar success in a patient with T1DM, results made possible in part by improved islet isolation techniques developed by Ricordi et al. [12]. The next major step was achieved with the introduction of steroid-sparing immunosuppressive therapy and modified islet isolation techniques, spearheaded by Shapiro's team [13] in Edmonton. The latter group reported that seven consecutive patients with T1DM were rendered insulin independent for a minimum of 1 year after receiving islet allografts, reflecting a success rate never previously achieved. Worldwide, more than 1000 individuals with T1DM have received allogeneic islet transplants since 1974. Although still a small number (especially when compared to more than 1 million afflicted with T1DM and an additional 18 million with T2DM in the US population, and the estimated 140 million with diabetes worldwide), much has been learned. The initial enthusiasm has been diminished by complications associated with the procedure itself, by concerns arising from the placement of allogeneic islets into the liver and, again, by the complications associated with the necessary immuno-suppression [14,15••].
Defining Success
The definition of successful islet transplant outcome has changed over time. In the mid-1990s, success was defined by graft function versus “no” graft function. In 1996, Luzi et al. [16] suggested surprisingly strict criteria for success: measurable C-peptide greater than 1.8 ng/mL, fasting plasma glucose less than 140 mg/dL, hemoglobin A1c less than 6.5%, and daily insulin doses of 0 to 8 U/d for at least 4 weeks. Using these criteria, only three of 15 patients (11 islet after kidney, one simultaneous islet/liver, three simultaneous islet/kidney) had a successful outcome. Another common definition of success has been insulin independence. Diem at al. [17] specified not only normoglycemia but also a biphasic insulin response to intravenous glucose stimulation. Similarly, in a recent review by Gruessner and Sutherland [1], a pancreas graft was defined as functioning as long as the patient was insulin independent. Even so, most investigators do no precisely define insulin independence, particularly with regard to the glycemia control they accept as normal, and when insulin therapy should be restarted. The criteria suggested by Ryan et al. [18••,19] are an exception. They define insulin independence as a state of no exogenous insulin use for 4 weeks with no more than two glucose values per week greater than 10 mmol/L (180 mg/dL) using the patients’ capillary glucose records. Insulin therapy was reinstituted when the fasting capillary glucose was greater than 8 mmol/L (144 mg/dL), the 2-hour postprandial glucose was greater than 10 mmol/L (180 mg/dL), and/or the hemoglobin A1c was consistently greater than 7%. Conversely, loss of islet graft function was defined as no detectable C-peptide (lower limit of detection 0.1 nmol/L) after mixed meal stimulations on two occasions or no detectable C-peptide with fasting glucose values greater than 15 mmol/L (270 mg/dL). Even these criteria raise eyebrows among endocrinologists. For instance, the ADA now defines a normal fasting glucose as less than 100 mg/dL and a normal hemoglobin A1c as less than 6.5%. Should islet transplant recipients not be considered for insulin even when the glycemia control is not normal? Our group initiated therapy with insulin sensitizers (metformin and thiazolidinediones) when fasting glucose concentrations were consistently above 100 mg/dL despite a hemoglobin A1c in the normal range. Further, we introduced insulin therapy if the former intervention did not consistently lower the fasting blood glucose levels below 100 mg/dL.
Islet Function Assays: Past and Present
Assessment of islet function in vivo has been difficult all along due to the lack of direct measures. Major efforts are underway to develop imaging techniques that would allow us to determine islet mass directly [20,21]. Absent the ability to directly measure islet cellular mass (or more precisely, β-cell mass within the islets), pancreas and islet transplant recipients are judged by their need for exogenous insulin, their glucose control, the frequency of hypoglycemia, and their endogenous insulin production, mostly determined by measuring circulating C-peptide concentrations. Specific considerations are worth emphasizing when evaluating the metabolic outcome of patients with systemically drained pancreas allografts. When insulin is secreted by the native pancreas into the portal vein, approximately 50% is cleared by the liver on first pass. However, a transplanted pancreas with venous blood routed into a systemic vein secretes insulin directly into the peripheral circulation, bypassing the liver and thus avoiding this first-pass effect. Regardless of the transplant procedure, C-peptide clearance may be prolonged by impaired kidney function [22]. Other obvious differences exist when assessing recipients of whole organ (pancreas) grafts compared with islet “mini-organs.” For instance, pancreas transplant recipients have serum exocrine function markers followed (because elevated amylase levels can indicate organ destruction), and for organs sewn to the bladder to handle pancreatic exocrine secretion, urinary amylase can serve as an indicator of the transplanted organ's health [23,24]. These techniques are useless for islet allograft recipients. Conversely, islet transplant recipients may undergo hepatic imaging and function studies to investigate the consequences of islets infused into the host's liver [25–29].
Most investigators have chosen one or more of several described metabolic tests to follow pancreas or islet allograft recipients. Examples include oral and intravenous glucose tolerance tests, mixed meal stimulation tests, glucagon and arginine (sometimes with “glucose potentiation”) stimulated C-peptide measurements, and hemoglobin A1c (Table 1) [13,14,16,17,18••,19,30–32,33•,34–37]. As so often in clinical practice, using the same test does not necessarily imply using the same technique, which can lead to inconsistent interpretations [13,14,17,18••,19,30,31,35]. Source documents that would be useful for characterizing specific tests are often underutilized due to time lapsed since the original publication. For example, the earliest descriptions of glucagon and arginine stimulation tests date back to the 1960s [38,39]. Additional measures of transplant outcome have included fasting glucose and C-peptide levels, the presence or absence of hypoglycemia, and exogenous insulin requirements.
Table 1.
Listing of various metabolic tests performed by leading investigators in the field of pancreas and islet transplantation
| Study (center) | Year | Patients, n | FU | OGTT/ IVGTT/clamp | GST/ AST/ MM | Fasting CP/ insulin/GIc | HbA1c | MAGE/HOMA/other |
|---|---|---|---|---|---|---|---|---|
| Islet transplantation | ||||||||
| Ricordi et al. [30]* (Pittsburgh/Miami) | 1992 | 25 | 5–16 mo | IVGTT | GST, MM | X | ||
| Luzi et al. [16] (Milan) | 1996 | 15 | > 4 wk | Clamp | X | FFA and lactate | ||
| Shapiro et al. [13] (Edmonton) | 2000 | 7 | 4.4–14.9 mo | OGTT | MM | Glc | X | GADA, IA-2A |
| Oberholzer et al. [31] (Geneva) | 2000 | 26 | 6 mo to 5 y | OGTT, IVGTT | GST | X | HOMA, GADA | |
| Luzi et al. [32] (Milan) | 2001 | 45 | 1–4 y | Clamp | All | X | Tracer study | |
| Ryan et al. [18••]† (Edmonton) | 2002 | 17 | 12–34 mo | OGTT, IVGTT | AST, MM | All | X | MAGE |
| Hirshberg et al. [14] (NIH) | 2003 | 6 | 17–22 mo | AST | X | |||
| Rickels et al. [33•] (Philadelphia) | 2005 | 5 | 3–12 mo | IVGTT, clamp | AST‡, MM | |||
| Ryan et al. [19] (Edmonton) | 2005 | 65 | 15 mo to 5 y | MM | Glc, insulin | X | HOMA, GADA, ICA, various scores | |
| Pancreas transplantation | ||||||||
| Diem et al. [17] | 1990 | 38 | 3–60 mo | AST | X | ITT | ||
| Blackman et al. [34] | 1992 | 10 | 6–24 mo | MM | X | X | Insulin pulses | |
| Robertson et al. [35] | 1999 | 96 | 3–60 mo | IVGTT | AST | Glc | X | |
| Kaufman et al. [36] | 2002 | 208 | Up to 8.5 y | X | ||||
| Dieterle et al. [37] | 2004 | 136 | 3–36 mo | OGTT | X |
Combination of two papers (Transplantation and Transplantation Proceedings) published in 1992.
Combination of two papers (Diabetes) published in 2001 and 2002.
Glc potentiated.
AST–arginine stimulation test; CP–C peptide; FFA–free fatty acid; FU–follow-up; CADA–glutamic acid decarboxylase antibodies; Glc–glucose; GST–glucagon stimulation test; HbA1c–hemoglobin A1c; HOMA–homeostasis model assessment; IA-2A–insulinoma-associated protein autoantibodies; ICA–islet cell autoantibodies; ITT–insulin tolerance test; IVGTT–intravenous glucose tolerance test; MAGE–mean amplitude of glycemie excursion; MM–mixed meal; NIH–National Institutes of Health; OGTT–oral glucose tolerance test.
Results of Individual Tests
For recipients of a pancreas transplant, available data usually show metabolic parameters that essentially mirror nondiabetic controls, except for increased basal insulin levels due to systemic drainage of the transplanted organ as mentioned earlier. For example, timing and amplitude of the acute insulin response to intravenous glucose were reported to be normal in 10 patients up to 18 years after the transplant procedure [35]. Similarly, the majority of successful pancreas transplant recipients have normal insulin, C-peptide, and glucose responses to oral glucose tolerance tests [17,34,37] and after challenge with a secretagogue [17,35].
In contrast, detailed follow-up of 17 islet transplant patients from Edmonton showed that only two had normal oral glucose tolerance test results 12 to 34 months after the procedure, whereas 11 had diabetes according to ADA criteria [18••]. Furthermore, islet transplant recipients typically have an absent or markedly reduced acute insulin response to intravenous glucose [16,18••,32,33•]. In the early days of islet transplantation, abnormal (delayed and prolonged) or absent C-peptide levels were frequently found in response to a mixed meal challenge associated with the known low success rate of the procedure [30,40]. In the modern era, up to 30 months after islet transplantation, C-peptide responses indistinguishable from nondiabetic controls were reported; however, they were paralleled by glucose values twice as high as in the controls [18••]. With longer follow-up, C-peptide responses decreased and glucose levels further increased [19]. In our group of six islet transplant recipients, two individuals had persistent basal and arginine stimulatable C-peptide up to 5 years after transplantation. Both individuals required small doses of long-acting insulin (glargine) to maintain normal blood glucose values. Edmonton has reported similar results in their much larger series, in that the acute insulin response to arginine persisted for prolonged periods, yet peak concentrations were always one third or less than achieved in healthy controls [18••]. Most investigators have not found evidence suggesting significant insulin resistance. For example, applying the homeostasis model assessment, originally developed to estimate β-cell function and insulin resistance in patients with T2DM, Ryan et al. [18••] found no significant changes over a follow-up period of up to 34 months. Using the euglycemic-hyperinsulinemic clamp technique, Luzi et al. [16] showed that patients with partially functioning grafts had higher basal hepatic glucose production and clearly defective tissue glucose disposal. Patients with fully functioning grafts (defined above) had normal basal hepatic glucose production, which, relative to nondiabetic controls, was only slightly less inhibited by insulin, such that glucose disposal was only mildly impaired [16]. Most within the islet transplant community agree that hypoglycemia frequency and blood glucose excursions are both markedly reduced. The latter have been quantified by using the mean amplitude of glycemic excursion score developed by Service et al. [41] in the late 1960s [18••].
Development of Special Indices and Composite Transplant Outcome Scores
Matsumoto et al. [42] have recommended an algorithm to assess islet engraftment. This index (secretory unit of islet transplant objects) is defined as follows: 1500 times fasting C-peptide immunoreactivity (ng/dL) divided by fasting blood glucose (mg/dL) minus 63. Ryan et al. [43] developed a similar index, or “beta score,” which uses a point value system consisting of the following: fasting glucose, hemoglobin A1c, mixed meal stimulated C-peptide levels, and use of insulin and/or oral hypoglycemic agents. The resulting score ranges from 0 to 8 (the higher the better) and was significantly correlated with glucose values at 90 minutes after ingestion of a mixed meal. To date, neither index is widely used in the transplant community.
Assessment of Islet Hormones Other than Insulin
Because islets consist of multiple different cell types secreting, in addition to insulin, other “old” hormones, such as glucagon, somatostatin, and pancreatic poly-peptide, as well as “new” hormones, such as ghrelin, clinical investigators have assessed their function in both islet and pancreas recipients. Many speculated, for instance, that islet transplant recipients are protected from severe hypoglycemia due to restored glucagon secretion from the islet allograft. Therefore, it was surprising to find that glucagon responses to hypoglycemia remain abnormal in islet transplant recipients [33•,44]. The islet recipients also display abnormal catecholamine and pancreatic polypeptide responses to hypoglycemia. Gupta et al. [45] hypothesized that the liver is an especially inadequate location for transplanted islets, because islets placed into the peritoneal cavity functioned well. The latter experiments were performed in dogs. Most now assume that islet recipients suffer less frequent severe hypoglycemia because of their reduced need for exogenous insulin.
Liver Imaging
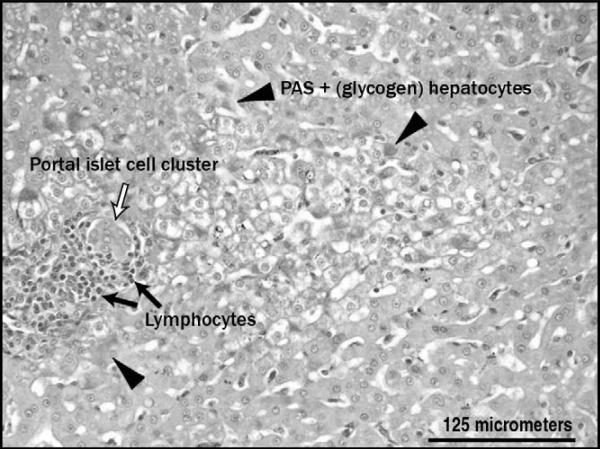
After we reported using our nonhuman primate model that intrahepatic islets influenced hepatic anatomy [46], several other groups published similar findings from clinical protocols. For instance, chemical shift MRI and ultrasound studies in humans have documented focal, periportal steatosis [25–28]. Various other techniques have been applied in animals to determine the fate (not the effect) of transplanted islets (eg, positron emission tomography, in vivo bioluminescence imaging of transplanted luciferase-expressing murine or human islets, and in vivo detection of islets labeled ex vivo with magnetic nanoparticles) [20,29,47]. Besides steatosis, our group also described local glycogen deposition in the nonhuman primate model (Fig. 1) [46]. Markmann et al. [28] questioned whether the observed fatty changes represented a “functional footprint of islet graft survival.” Maffi et al. [27] also linked the focal areas of hyperechogenicity, which they observed using liver ultrasound, with partial function of islet grafts (transient insulin independence with prolonged C-peptide secretion). None of the individuals with undetectable C-peptide and few insulin-independent patients had such changes suggestive of fatty liver. Thus, it has been hypothesized that ailing islets may possibly cause hepatic abnormalities due to a combination of local insulin effects and an altered cytokine milieu. This hypothesis warrants further study, as does the recent provocative report suggesting a heightened risk for hepatocellular carcinoma in a rat islet transplant model [48].
Figure 1.
Local glycogen deposition surrounding an intrahepatic islet allograft in a nonhuman primate. PAS—periodic acid–Schiff.
Can Successful Outcome of Transplantation Be Predicted?
Most investigators in the field of islet transplantation agree that there is as yet little correlation between various islet function tests performed after islet isolation, before islet transplantation, and the patient's subsequent clinical outcome. Islet viability and function can be affected by many factors, including donor characteristics, the duration of cold ischemia prior to islet isolation, the isolation process itself, and the culture period before transplantation. Several methods have been studied, for example tests of membrane integrity, oxygen consumption, insulin secretion capacity, mitochondrial activity, and gene profiling [49–52]. In most cases, either the assay has a poor predictive value, or the test takes too much time for clinical use.
Obstacles
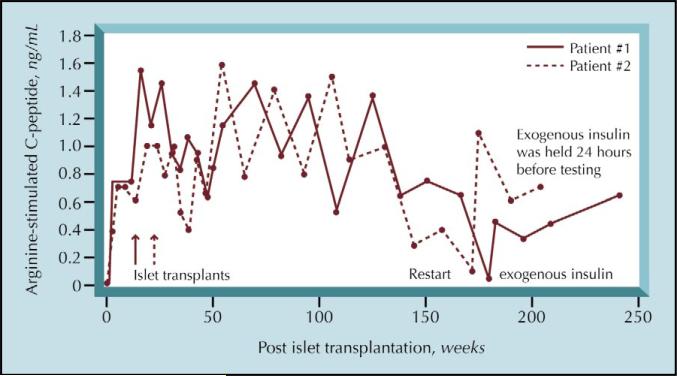
Assessing β-cell function in insulin-independent post-transplant patients using insulin assays requires great caution because insulin is secreted via a nonphysiologic route in systemically drained pancreas allografts and in hepatic islet allografts, thus complicating insulin secretion rate interpretations. When using C-peptide levels to assess islet function, kidney function plays an important role. Renal insufficiency impairs C-peptide excretion and thus prolongs its half-life. Among others, Christiansen et al. [22] and Blackman et al. [34] have developed mathematical models designed to avoid mis-interpretation. But these deconvolution methods are not easily nor widely performed [22,34]. In addition, we have found that stimulated C-peptide results from insulin-requiring islet transplant recipients with partial allograft function are heavily influenced by timing and dose of exogenous insulin (Fig. 2). Taking these caveats into account, overall insulin secretion rates are similar in patients receiving both a pancreas and kidney transplant, compared with individuals who received only a kidney (control patients) [34]. However, the relationship between basal and meal-stimulated insulin secretion was altered with increased basal insulin and reduced meal-related secretion in the combined organ recipients.
Figure 2.
Two National Institutes of Health patients, who received islet allografts 5 years prior, have persistent C-peptide secretion up to the present time. Depicted are their C-peptide responses (defined as the maximum C-peptide concentration [mean of the highest three values after arginine stimulation] minus the mean of two baseline values). After introduction of exogenous long-acting insulin, decreased C-peptide responses were observed and restored after omission of insulin 24 hours prior to testing.
Using C-peptide levels as an indicator of functional β-cell mass is another delicate issue. Teuscher et al. [53] found a good correlation between the glucose-potentiated insulin response to arginine and transplanted islet mass in eight nondiabetic autograft islet recipients. Many investigators have thus extrapolated these findings with the assumption that they apply when using arginine-stimulated C-peptide results (instead of insulin levels) in islet allograft (instead of autograft) recipients. Furthermore, no standard C-peptide assay exists, but various assays are used with different cross-reactivity and lower limits of detection (typically between 0.05 and 0.5 ng/mL).
Conclusions
What is the gold standard for assessment of islet function after whole organ pancreas or isolated islet transplantation? There is no gold standard. Each of the currently available tests is an indirect or deduced measure of islet mass and function, and is based on patient populations other than transplant recipients. Even so, scientifically interesting and clinically important information has been gained by performing oral and intravenous glucose tolerance tests, stimulation of insulin secretion with test meals and secretagogues, to name a few. We need improved and direct methods to better understand the survival, possible regeneration, health, and function of pancreatic islets.
Acknowledgement
This research was supported in part by the Intramural Research Programs of the NIDDK, NIH, and DHHS.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Gruessner AC, Sutherland DE. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin Transplant. 200519:433–455. doi: 10.1111/j.1399-0012.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland DE, Gruessner RW, Dunn DL, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson RP, Davis C, Larsen J, et al. Pancreas transplantation for patients with type 1 diabetes. Diabetes Care. 2003;26(suppl 1):S120. doi: 10.2337/diacare.26.2007.s120. [DOI] [PubMed] [Google Scholar]
- 4.Landgraf R. Impact of pancreas transplantation on diabetic secondary complications and quality of life. Diabetologia. 1996;39:1415–1424. doi: 10.1007/s001250050593. [DOI] [PubMed] [Google Scholar]
- 5.Venstrom JM, McBride MA, Rother KI, et al. Survival after pancreas transplantation in patients with diabetes and preserved kidney function. JAMA. 2003;290:2817–2823. doi: 10.1001/jama.290.21.2817. [DOI] [PubMed] [Google Scholar]
- 6.Joist H, Brennan DC, Coyne DW. Anemia in the kidney-transplant patient. Adv Chronic Kidney Dis. 2006;13:4–10. doi: 10.1053/j.ackd.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Boratynska M, Banasik M, Watorek E, et al. Conversion to sirolimus from cyclosporine may induce nephrotic proteinuria and progressive deterioration of renal function in chronic allograft nephropathy patients. Transplant Proc. 2006;38:101–104. doi: 10.1016/j.transproceed.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Williams D, Haragsim L. Calcineurin nephrotoxicity. Adv Chronic Kidney Dis. 2006;13:47–55. doi: 10.1053/j.ackd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Najarian JS, Sutherland DE, Matas AJ, et al. Human islet transplantation: a preliminary report. Transplant Proc. 1977;9:233–236. [PubMed] [Google Scholar]
- 10.Largiader F, Kolb E, Binswanger U. A long-term functioning human pancreatic islet allotransplant. Transplantation. 1980;29:76–77. doi: 10.1097/00007890-198001000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Scharp DW, Lacy PE, Santiago JV, et al. Insulin independence after islet transplantation into type I diabetic patient. Diabetes. 1990;39:515–518. doi: 10.2337/diab.39.4.515. [DOI] [PubMed] [Google Scholar]
- 12.Ricordi C, Lacy PE, Finke EH, et al. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 14.Hirshberg B, Rother KI, Digon BJ, III, et al. Benefits and risks of solitary islet transplantation for type 1 diabetes using steroid-sparing immunosuppression: the National Institutes of Health experience. Diabetes Care. 2003;26:3288–3295. doi: 10.2337/diacare.26.12.3288. [DOI] [PubMed] [Google Scholar]
- 15••.Rother KI, Harlan DM. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest. 2004;114:877–883. doi: 10.1172/JCI23235. [Perspective of progress as well as remaining challenges in the field of islet cell transplantation and replacement.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luzi L, Hering BJ, Socci C, et al. Metabolic effects of successful intraportal islet transplantation in insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:2611–2618. doi: 10.1172/JCI118710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diem P, Redmon JB, Abid M, et al. Glucagon, catecholamine and pancreatic polypeptide secretion in type I diabetic recipients of pancreas allografts. J Clin Invest. 1990;86:2008–2013. doi: 10.1172/JCI114936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Ryan EA, Lakey JR, Paty BW, et al. Successful islet transplantation: continued insulin reserve provides long-term glycemic control. Diabetes. 2002;51:2148–2157. doi: 10.2337/diabetes.51.7.2148. [Excellent overview of the first 3 years after islet transplantation.] [DOI] [PubMed] [Google Scholar]
- 19.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060–2069. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 20.Evgenov NV, Medarova Z, Dai G, et al. In vivo imaging of islet transplantation. Nat Med. 2006;12:144–148. doi: 10.1038/nm1316. [DOI] [PubMed] [Google Scholar]
- 21.Paty BW, Bonner-Weir S, Laughlin MR, et al. Toward development of imaging modalities for islets after transplantation: insights from the National Institutes of Health Workshop on Beta Cell Imaging. Transplantation. 2004;77:1133–1137. doi: 10.1097/01.tp.0000113231.90613.0e. [DOI] [PubMed] [Google Scholar]
- 22.Christiansen E, Kjems LL, Volund A, et al. Insulin secretion rates estimated by two mathematical methods in pancreas-kidney transplant recipients. Am J Physiol. 1998;274(4 Pt 1):E716–E725. doi: 10.1152/ajpendo.1998.274.4.E716. [DOI] [PubMed] [Google Scholar]
- 23.Sutherland DE, Gruessner A, Hering BJ. Beta-cell replacement therapy (pancreas and islet transplantation) for treatment of diabetes mellitus: an integrated approach. Endocrinol Metab Clin North Am. 2004;33:135–148. x. doi: 10.1016/S0889-8529(03)00099-9. [DOI] [PubMed] [Google Scholar]
- 24.Robertson RP, Sutherland DE, Kendall DM, et al. Metabolic characterization of long-term successful pancreas transplants in type I diabetes. J Investig Med. 1996;44:549–555. [PubMed] [Google Scholar]
- 25.Bhargava R, Senior PA, Ackerman TE, et al. Prevalence of hepatic steatosis after islet transplantation and its relation to graft function. Diabetes. 2004;53:1311–1317. doi: 10.2337/diabetes.53.5.1311. [DOI] [PubMed] [Google Scholar]
- 26.Eckhard M, Lommel D, Hackstein N, et al. Disseminated periportal fatty degeneration after allogeneic intraportal islet transplantation in a patient with type 1 diabetes mellitus: a case report. Transplant Proc. 2004;36:1111–1116. doi: 10.1016/j.transproceed.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 27.Maffi P, Angeli E, Bertuzzi F, et al. Minimal focal steatosis of liver after islet transplantation in humans: a long-term study. Cell Transplant. 2005;14:727–733. doi: 10.3727/000000005783982567. [DOI] [PubMed] [Google Scholar]
- 28.Markmann JF, Rosen M, Siegelman ES, et al. Magnetic resonance-defined periportal steatosis following intraportal islet transplantation: a functional footprint of islet graft survival? Diabetes. 2003;52:1591–1594. doi: 10.2337/diabetes.52.7.1591. [DOI] [PubMed] [Google Scholar]
- 29.Toso C, Zaidi H, Morel P, et al. Positron-emission tomography imaging of early events after transplantation of islets of Langerhans. Transplantation. 2005;79:353–355. doi: 10.1097/01.tp.0000149501.50870.9d. [DOI] [PubMed] [Google Scholar]
- 30.Ricordi C, Tzakis AG, Carroll PB, et al. Human islet isolation and allotransplantation in 22 consecutive cases. Transplantation. 1992;53:407–414. doi: 10.1097/00007890-199202010-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oberholzer J, Triponez F, Mage R, et al. Human islet transplantation: lessons from13 autologous and 13 allogeneic transplantations. Transplantation. 2000;69:1115–1123. doi: 10.1097/00007890-200003270-00016. [DOI] [PubMed] [Google Scholar]
- 32.Luzi L, Perseghin G, Brendel MD, et al. Metabolic effects of restoring partial beta-cell function after islet allotransplantation in type 1 diabetic patients. Diabetes. 2001;50:277–282. doi: 10.2337/diabetes.50.2.277. [DOI] [PubMed] [Google Scholar]
- 33•.Rickels MR, Schutta MH, Markmann JF, et al. Beta-cell function following human islet transplantation for type 1 diabetes. Diabetes. 2005;54:100–106. doi: 10.2337/diabetes.54.1.100. [Evaluation of multiple islet hormones in addition to insulin in islet transplant patients.] [DOI] [PubMed] [Google Scholar]
- 34.Blackman JD, Polonsky KS, Jaspan JB, et al. Insulin secretory profiles and C-peptide clearance kinetics at 6 months and 2 years after kidney-pancreas transplantation. Diabetes. 1992;41:1346–1354. doi: 10.2337/diab.41.10.1346. [DOI] [PubMed] [Google Scholar]
- 35.Robertson RP, Sutherland DE, Lanz KJ. Normoglycemia and preserved insulin secretory reserve in diabetic patients 10-18 years after pancreas transplantation. Diabetes. 1999;48:1737–1740. doi: 10.2337/diabetes.48.9.1737. [DOI] [PubMed] [Google Scholar]
- 36.Kaufman DB, Leventhal JR, Gallon LG, et al. Technical and immunologic progress in simultaneous pancreas-kidney transplantation. Surgery. 2002;132:545–553. doi: 10.1067/msy.2002.127547. [DOI] [PubMed] [Google Scholar]
- 37.Dieterle CD, Schmauss S, Veitenhansl M, et al. Glucose metabolism after pancreas transplantation: cyclosporine versus tacrolimus. Transplantation. 2004;77:1561–1565. doi: 10.1097/01.tp.0000129063.65446.65. [DOI] [PubMed] [Google Scholar]
- 38.Dupre J, Curtis JD, Unger RH, et al. Effects of secretin, pancreozymin, or gastrin on the response of the endocrine pancreas to administration of glucose or arginine in man. J Clin Invest. 1969;48:745–757. doi: 10.1172/JCI106032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet. 1965;40:415–416. doi: 10.1016/s0140-6736(65)90761-0. [DOI] [PubMed] [Google Scholar]
- 40.Alejandro R, Lehmann R, Ricordi C, et al. Long-term function (6 years) of islet allografts in type 1 diabetes. Diabetes. 1997;46:1983–1989. doi: 10.2337/diab.46.12.1983. [DOI] [PubMed] [Google Scholar]
- 41.Service FJ, Molnar GD, Rosevear JW, et al. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto S, Yamada Y, Okitsu T, et al. Simple evaluation of engraftment by secretory unit of islet transplant objects for living donor and cadaveric donor fresh or cultured islet transplantation. Transplant Proc. 2005;37:3435–3437. doi: 10.1016/j.transproceed.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 43.Ryan EA, Paty BW, Senior PA, et al. Beta-score: an assessment of beta-cell function after islet transplantation. Diabetes Care. 2005;28:343–347. doi: 10.2337/diacare.28.2.343. [DOI] [PubMed] [Google Scholar]
- 44.Paty BW, Ryan EA, Shapiro AM, et al. Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes. 2002;51:3428–3434. doi: 10.2337/diabetes.51.12.3428. [DOI] [PubMed] [Google Scholar]
- 45.Gupta V, Wahoff DC, Rooney DP, et al. The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes. 1997;46:28–33. doi: 10.2337/diab.46.1.28. [DOI] [PubMed] [Google Scholar]
- 46.Hirshberg B, Mog S, Patterson N, et al. Histopathological study of intrahepatic islets transplanted in the nonhuman primate model using Edmonton protocol immunosuppression. J Clin Endocrinol Metab. 2002;87:5424–5429. doi: 10.1210/jc.2002-020684. [DOI] [PubMed] [Google Scholar]
- 47.Fowler M, Virostko J, Chen Z, et al. Assessment of pancreatic islet mass after islet transplantation using in vivo bioluminescence imaging. Transplantation. 2005;79:768–776. doi: 10.1097/01.tp.0000152798.03204.5c. [DOI] [PubMed] [Google Scholar]
- 48.Dombrowski F, Mathieu C, Evert M. Hepatocellular neoplasms induced by low-number pancreatic islet transplants in autoimmune diabetic BB/Pfd rats. Cancer Res. 2006;66:1833–1843. doi: 10.1158/0008-5472.CAN-05-2787. [DOI] [PubMed] [Google Scholar]
- 49.Ichii H, Inverardi L, Pileggi A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5:1635–1645. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 50.Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation. 1988;45:827–830. [PubMed] [Google Scholar]
- 51.Sabek OM, Marshall DR, Minoru O, et al. OP-142 gene expression profile of nonfunctional human pancreatic islets: predictors of transplant failure? Transplant Proc. 2005;37:3441–3443. doi: 10.1016/j.transproceed.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 52.Sweet IR, Khalil G, Wallen AR, et al. Continuous measurement of oxygen consumption by pancreatic islets. Diabetes Technol Ther. 2002;4:661–672. doi: 10.1089/152091502320798303. [DOI] [PubMed] [Google Scholar]
- 53.Teuscher AU, Kendall DM, Smets YF, et al. Successful islet autotransplantation in humans: functional insulin secretory reserve as an estimate of surviving islet cell mass. Diabetes. 1998;47:324–330. doi: 10.2337/diabetes.47.3.324. [DOI] [PubMed] [Google Scholar]