Fig. 8.
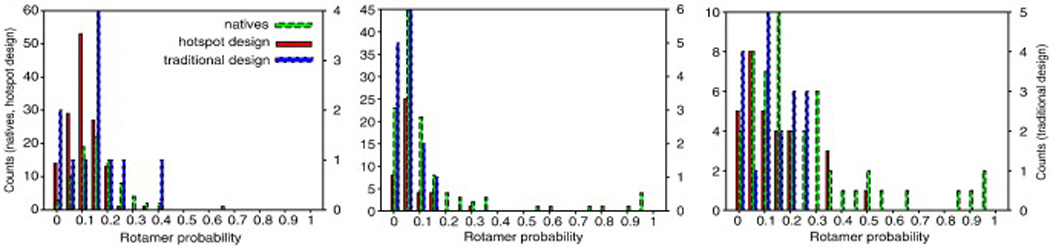
Comparison of side-chain conformational probabilities in natural and designed complexes. The side-chain conformational probabilities in the unbound state [Eq. (3)] were computed using the method in Ref. 20. Hotspot-centric design yields complexes with a comparable proportion of low-probability conformations (≤ 0.05 probability) to native complexes, whereas the “traditional designs” selected on the basis of binding affinity show proportionately more low-probability side-chain conformations. Both design strategies have fewer high-probability conformations (>0.50) than do natural interfaces, potentially explaining the low success rate in the protein binder design. The energetic consequences of differences in the intermediate probability range (0.1–0.5) are less significant than in the extremes of the probability. The native set contains the docking benchmark complexes (120 proteins);52 the hotspot designed set is taken from the protein-interface design benchmark (87 proteins) (Fleishman et al., in press), and the traditional designs are taken from Ref. 20.
