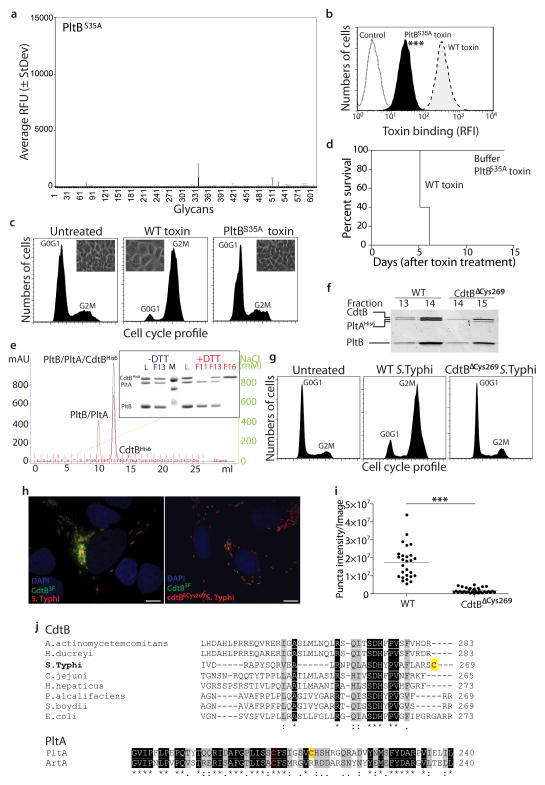
Figure 4.
Structure-function analysis of typhoid toxin. a–d, Fluorescently-labeled typhoid toxin containing PltBSer35A was tested for its binding to glycans (a) and to cultured cells (b) (see Fig. 2 for details) (***, P < 0.001 from at least three independent determinations). Alternatively, toxicity was assayed by flow cytometric cell cycle analysis of toxin-treated cells (at least three independent experiments) (c), or by systemic administration to mice (n=3 to 5 mice) (d). e–i, The typhoid toxin complex was analyzed by ion exchange chromatography before (blue) and after (red) treatment with DTT (L: loading control; M: molecular weight markers; F: chromatographic fraction) (e). Inset shows SDS-PAGE analyzes of the indicated fractions (e). f, A toxin preparation obtained from a bacterial strain expressing CdtBΔcys269 was analyzed by gel filtration chromatography and compared to wild-type toxin (the experiment was repeated two times). While wild-type holotoxin eluted in fractions 13 and 14, toxin obtained from a bacterial strain encoding CdtBΔcys269 eluted in fractions 14 and 15 due to the lack of CdtB. g–i, Henle-407 cells were infected with S. Typhi strains encoding FLAG-tagged CdtB or CdtBΔcys269 and cells were examined for toxicity by flow cytometry (g). Alternatively, cells were fixed, stained with anti FLAG antibody, and the amount of puncta staining, which represent CdtB in typhoid toxin export carriers8, were determined by immunofluorescence analysis (h and i). Bar represents average of puncta-associated flurorescence intensity (at least 100 cells were analyzed in three independent experiments).***, P < 0.0001, Scale bar:10 μm. j, ClustalW amino acid sequence comparison of CdtB and PltA homologs. Conserved cysteines are shown in red while unique cysteines are indicagted with a yellow shade.