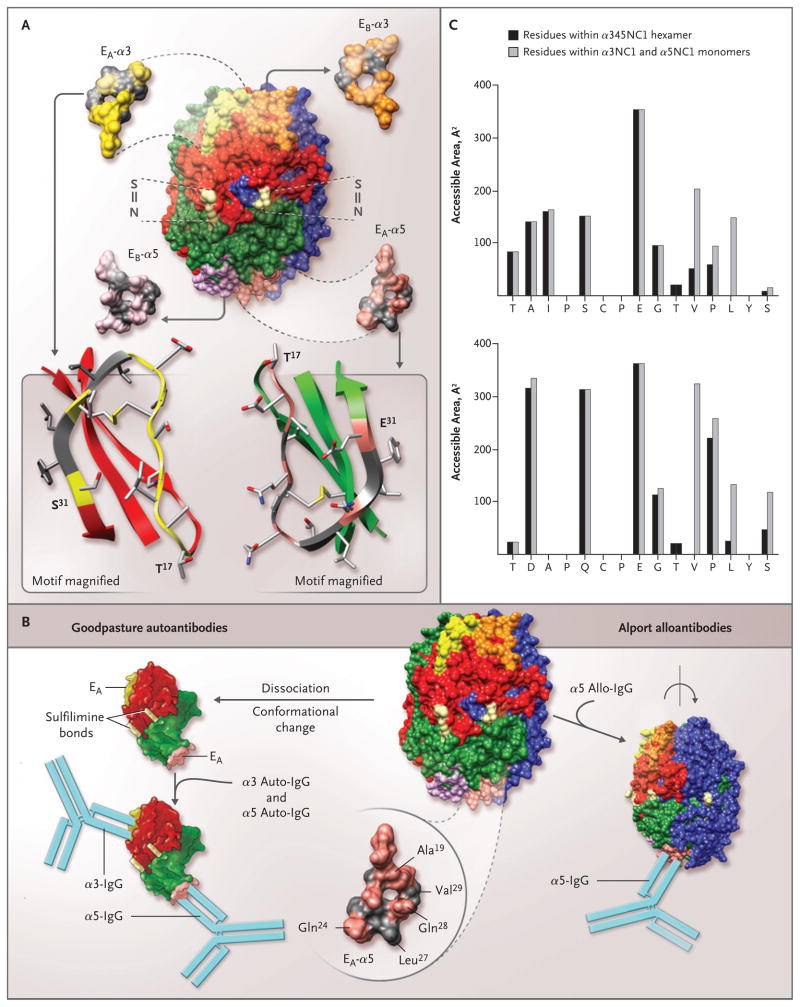
Figure 4. Topology of the EA and EB Regions in the α345 Noncollagenous-1 Hexamer, Structural Determinants for the Binding of Alport Alloantibodies and Goodpasture Autoantibodies In Vitro, and Accessible Surface Area of the EA-α3 and EA-α5 Regions of the Noncollagenous-1 Hexamer.
The α345 noncollagenous-1 (NC1) hexamer is composed of two trimeric caps, each consisting of α3NC1 (red), α4NC1 (blue), and α5NC1 (green) subunits (Panel A). Two of the six sulfilimine bonds (S = N) that stabilize the trimer–trimer interface are shown (light yellow). The location and structure of the four homologous regions are also shown: EA (yellow) and EB (orange) in the α3NC1 subunit, and EA (pink) and EB (purple) in the α5NC1 subunit. Three regions, EA and EB in α3NC1 and EA in α5NC1, become critical parts of the neoepitopes for Goodpasture autoantibodies. The topology of the EA regions in α3NC1 and α5NC1 is similar, as indicated in the ribbon diagrams (Panel A, bottom), with the characteristic folding pattern of a β-sheet stabilized with a disulfide bond. Ala19, Gln24, and Gln28 (pink) within the EA region of α5NC1, exposed in the α345NC1 hexamer, are candidates for the binding of Alport alloantibodies (Panel B, bottom right). In contrast, Leu27 and Val29 (gray) are sequestered by their lateral interaction with the α4NC1 domain, and when exposed as a result of hexamer dissociation, they become critical to the binding of Goodpasture autoantibodies. Dissociation of the sulfilimine-cross-linked hexamer into α35 dimer subunits is concomitant with a conformational change that results in the formation of the neoepitopes encompassing the EA regions of the α5NC1 and α3NC1 monomers and the binding of their respective autoantibodies (Panel B, bottom left). The accessible surface area of the EA-α3 region (Panel C, top) and the EA-α5 region (Panel C, bottom) was calculated for a probe, which mimics the antibody molecule (radius, 9 Å); the area of individual residues in the α345NC1 hexamer (black bars) and the α3NC1/α5NC1 model monomers (gray bars) is shown. An increase in the surface area of the monomers indicates that residues are buried in the hexamer (Val27 and Leu29 in EA-α3 and Leu27 and Val29 in EA-α5). In contrast, residues with similar areas within the hexamer and monomers are exposed in the hexamer (Ala19, Gln24, and Gln28 in EA-α5).