SUMMARY
Listeria monocytogenes infected CD8α+ DCs in the spleen are essential for CD8+ T cell generation. CD8α+ DCs are also necessary for Listeria expansion and dissemination within the host. The mechanisms that regulate CD8α+ DCs to allow Listeria expansion are unclear. We find that activating the B and T lymphocyte attenuator (BTLA), a co-inhibitory receptor on CD8α+ DCs, suppresses, while blocking BTLA enhances both the primary and memory CD8 T cell responses against Listeria. Btla−/− mice have lower effector and memory CD8+ T cells while paradoxically also being more resistant to Listeria. Although bacterial entry into Btla−/− CD8α+ DCs is unaffected, Listeria fails to expand within these cells. BTLA signaling limits Fas/FasL-mediated suppression of Listeria expansion within CD8α+ DCs to more effectively alert adaptive immune cells. This study uncovers a BTLA-mediated strategy used by the host that permits Listeria proliferation to enable increasing T cell responses for long-term protection.
INTRODUCTION
Listeria monocytogenes is a gram-positive facultative intracellular bacterial pathogen. It is the causative agent of Listeriosis that often affects unborn fetuses and immunocompromised individuals (Dussurget et al., 2004; Kaufmann, 1993). Listeria-infected animal models are common infectious models to study systemic immune responses to intracellular pathogens, as defined innate, effector, and memory responses are essential to limit Listeria infection (Pamer, 2004). Without an effective adaptive response to Listeria, infected mice are not able to survive. This was originally demonstrated by showing that immunodeficient nude, SCID, and Rag1−/− mice developed a chronic Listeria infection and eventually died, while immunocompetent WT mice cleared the infection (Bancroft et al., 1986; Emmerling et al., 1975; Mombaerts et al., 1993). Interestingly, immunodeficient animals harbor lower CFU levels of splenic Listeria than immunocompetent mice during the early phase of infection, indicating that the innate immune response could function independently of the adaptive immune response to limit early infection (Carrero et al., 2006; Emmerling et al., 1975). This result further indicated that the adaptive immune response directly or indirectly regulates the amplitude of the innate immune response.
As for host adaptive immunity to Listeria, a body of literature describes the interaction between Listeria infection and the generation of bacterial-specific CD8+ T cells. Indeed, CD8+ T cells are required for sterilizing immunity since β2M−/− mice have shown delayed Listeria clearance (Roberts et al., 1993). Of the CD8+ T cell functions required for sterilizing immunity, the most important is the CD8+ T cell cytotoxic function, as perforin-deficient CD8+ T cells cannot control Listeria infection (Jensen et al., 1998; Kagi et al., 1994; Mittrucker et al., 2000; San Mateo et al., 2002). Upon secondary infection, the ability of the immune system to rapidly sterilize the host from Listeria infection is largely dependent upon the memory CD8+ T cell recall response (Harty et al., 1992). Listeria infection also induces CD4 T cell responses, which can help host to control the infection: depleting CD4 T cells will reduce the host immunity against Listeria infection, while adoptive transferring CD4 T cells from infected mice to naive mice can provide partial protection (Baldridge et al., 1990; Kaufmann, 1993; Kaufmann et al., 1985; Ladel et al., 1994; Mittrucker et al., 2000). The role of innate cells during secondary or memory responses is considered minimum. However, recent publication showed that the recall response after second Listeria infection was highly orchestrated by both memory CD8+ T and innate cells: inflammatory Ly6C+ monocytes activate memory CD8+ T and NK lymphocytes(Soudja et al., 2012) and cytotoxic memory CD8+ T cells can license inflammatory monocytes and neutrophils to clear Listeria infection (Narni-Mancinelli et al., 2011). Interestingly, the generation of effective CD8+ memory T cells is dependent upon infection with live but not heat-killed Listeria (Datta et al., 2006); Listeria needs to enter the host cell cytosol in order to generate an effective memory response, as demonstrated by LLO-deficient Listeria that are unable to induce protective immunity (Berche et al., 1987).
Recently publication has shown that platelet is required for shuttling Listeria to splenic CD8α+ DCs (Verschoor et al., 2011). Splenic CD8α+ DCs localize to the marginal zone and efficiently cross-present viral and intracellular bacterial antigens to CD8+ T cells (Hildner et al., 2008). These CD8α+ DCs are uniquely dependent upon the transcription factor Batf3 for their development (Hildner et al., 2008). The essential role of splenic CD8α+ DCs in CD8+ T cell generation upon Listeria infection has been demonstrated (Campisi et al., 2011; Mitchell et al., 2011). However, recent studies have shown that they are also necessary for harboring and disseminating Listeria within the host (Edelson et al., 2011; Neuenhahn et al., 2006). In the spleen, CD8α+ DCs are the first cells infected with Listeria as early as 1 hour after infection (Verschoor et al., 2011). Confirming that CD8α+ DCs were necessary for major Listeria expansion and dissemination, Batf3−/− mice were recently demonstrated to have much lower Listeria burden after infection (Edelson et al., 2011). Compared with macrophage, CD8α+ DCs DCs are considered less armed for directly fighting against bacterial or viral infection but can fascinatingly control listerial proliferation. What molecular mechanisms regulate function of these cells to allow Listeria expansion, however, is still unclear.
BTLA signaling in T cells appears to limit T cell responses. We initially reported that the HVEM delivers signals to suppress T cell responses and that Hvem−/− mice are prone to autoimmunity (Wang et al., 2005). Others reported that HVEM partnered with BTLA inhibited the adaptive immune system (Krieg et al., 2007; Sedy et al., 2005). HVEM-BTLA interactions also regulate early immunity through regulating NKT function because both Hvem−/− and Btla−/− mice are hyper-responsive to NKT-dependent Con A challenge compared to WT mice (Miller et al., 2009; Wang et al., 2005). We recently observed that Hvem−/− and Btla−/− mice have reduced bacterial burden compared to WT mice in the liver and spleen within 4 days of Listeria infection (Sun et al., 2009). In this study, Btla−/− mice showed reduced primary and secondary CD8+ T cell responses upon Listeria infection while being more resistant to Listeria infection. Rather than directly limiting T cell responses against Listeria infection, we have now revealed the dominant role of BTLA signaling during Listeria infection is to permit the expansion of Listeria in CD8α+ DCs in order to induce stronger priming of T cells.
RESULTS
BTLA signaling inhibits Listeria-specific CD8+ effector and memory T cell responses
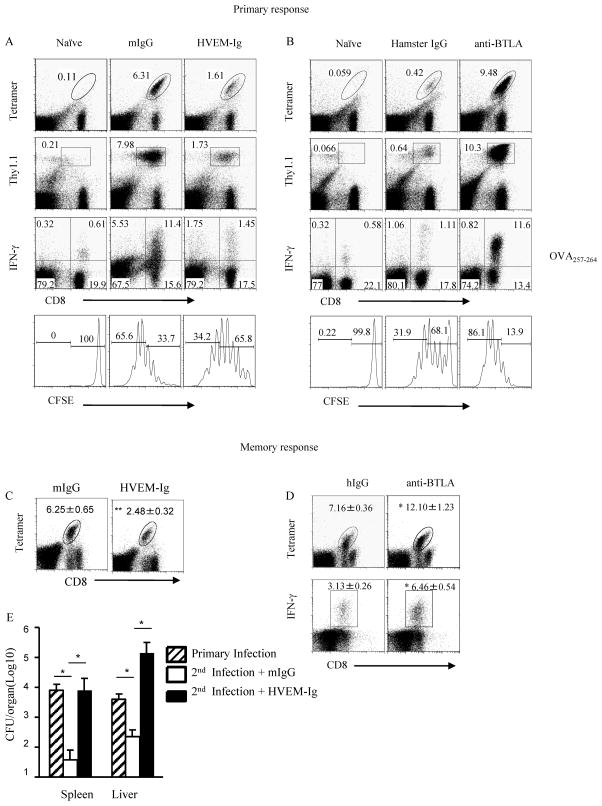
Previous studies have defined BTLA as a co-inhibitory receptor in T cells that inhibits T cell function through its immunoreceptor tyrosine-based inhibitory motifs (ITIMs) (Krieg et al., 2007; Sedy et al., 2005; Watanabe et al., 2003). Our previous data showed that WT mice treated with the BTLA agonist, HVEM-Ig, had 2 to 3 logs higher levels of Listeria CFU 3 days after infection, whereas the antagonistic anti-BTLA mAb consistently showed suppressed CFU levels, suggesting that the BTLA pathway inhibits the clearance of Listeria infection (Sun et al., 2009). BTLA-dependent control of Listeria could be attributed to both innate and adaptive immunity. However, it is unclear whether BTLA regulated Listeria proliferation directly. To test whether BTLA inhibited CD8+ T cell activity, Listeria-OVA infected mice were treated with HVEM-Ig to cross-link BTLA, thereby triggering the BTLA downstream signaling. OVA-specific T cells and the IFNγ production were decreased in mice treated with the BTLA agonist, HVEM-Ig (Figure 1A and Figure S1A). To test whether a BTLA antagonist would increase the T cell response, we infected mice with low dosage of Listeria to better observe an increased responses. Indeed, bacterial specific CD8+ T cells and the IFNγ production were increased in mice treated with the anti-BTLA blocking mAb (Figure 1B and Figure S1B). Therefore, the data suggest that BTLA signaling in T cells is likely inhibitory, suppressing T cell proliferation during Listeria infection.
Figure 1.
BTLA inhibits bacteria-specific CD8+ effector and memory T cell responses. A) CFSE-labeled pooled splenocytes and LN cells isolated from OTI/Thy1.1 mice (2×107 cells) were adoptively transferred into WT Thy1.2+ mice, and the recipients were immunized with 5×105 CFU Listeria-OVA and treated with 200 μg HVEM-Ig or mIgG as control. Seven days later, splenocytes were isolated and Thy1.1+ CD8+ OVA -tetramer+ cells and intracellular IFNγ were analyzed by flow cytometry. B) CFSE-labeled pooled splenocytes and LN cells isolated from OTI/Thy1.1 mice (2×107 cells) were adoptively transferred into WT mice, and the recipients were immunized with low dosage 1×105 CFU Listeria-OVA and treated with 200 μg anti-BTLA (6A6) or hamster IgG. Seven days later, splenocytes were isolated and Thy1.1+ CD8+ OVA-tetramer+ cells and intracellular IFNγ were analyzed by flow cytometry. C) WT mice were primarily infected i.p. with 1×104 CFU Listeria-OVA. Two months later, mice were re-challenged with 5×106 CFU Listeria-OVA and treated with 200 μg HVEM-Ig or mIgG (C), Hamster Ig or anti-BTLA (6A6) Ab (D). Six days later, splenocytes were isolated and endogenous CD8+ OVA -tetramer+ cells and intracellular IFNγ were analyzed by flow cytometry. E) Same as in C, three days after rechallenge the bacteria burden in the spleen and liver was tested. The results are representative of three independent experiments, four to five mice in each group and data are represented as mean +/− SEM. See also Figure S1.
The memory response to Listeria infection is primarily comprised of the CD8+ T cell and plays a dominant role for clearance of pathogens. Because BTLA has been shown to inhibit the CD8+ effector response, we expected that BTLA would similarly inhibit the CD8+ T cell memory response. Indeed, memory CD8+ T cell responses to Listeria infection were severely diminished after HVEM-Ig cross-linking of BTLA, and were significantly enhanced after BTLA blockade during Listeria second infection (Figure 1C, D and Figure S1C). Additionally, the Listeria burden corresponded to the changes in T cell responses, with reduced memory T cell responses corresponding to greater Listeria burden, and vice versa, increased memory T cell responses corresponding to much lower bacterial burden (Figure 1E). This result matches well with the established dogma that CD8+ T cells play a major role in controlling Listeria burden during both primary and secondary infection and further suggests the inhibitory role of BTLA in T cells during primary and secondary Listeria infection.
Anti-Listiera CD8+ T cell responses are unexpectedly reduced in Btla−/− mice
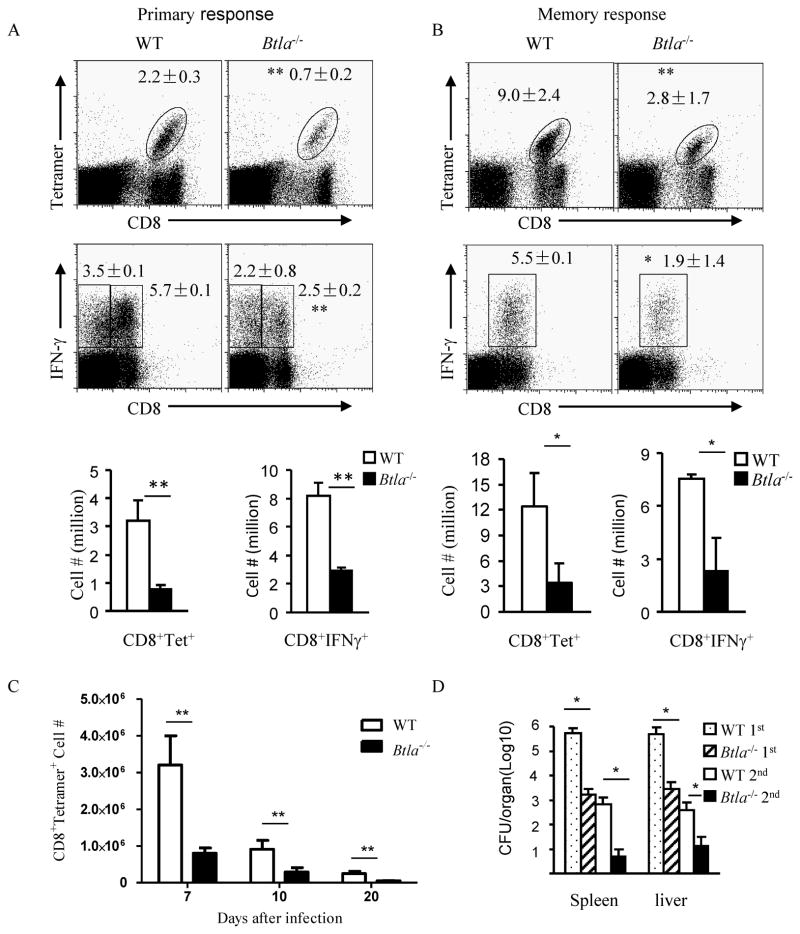
While transferring to the same WT recipient mice, BTLA deficient T cells are more active and proliferate more than WT T cells in response to Listeria infection (Krieg et al., 2007). Together with other studies, it is expected that Btla−/− mice should have increased CD8+ T cell responses during Listeria infection as compared to WT mice. Surprisingly, we observed that Btla−/−− mice actually generated significantly fewer CD8+ T cell responses not only during the primary response, but also during the secondary response to pathogen rechallenge (Figure 2A–B). During different time points after infection, we have observed the similar reduction of T cell response in Btla−/− mice (Figure 2C). Since HVEM is the only known binding partner for BTLA, we tested whether Hvem−/− mice have similar reduction of T cell responses. Indeed, we observed that T cell generation in Hvem−/− mice is similarly reduced after Listeria infection (Data not shown). In naive mice, there is no difference in CD8 T cell number between WT and Btla−/− or Hvem−/− mice, suggesting no pre-existing deficiency in CD8 T cell numbers (Krieg et al., 2007). Compared to WT mice, Btla−/− mice demonstrated a much lower Listeria burden concurrent with the lower CD8+ T cell profile during primary responses (Figure 2D).
Figure 2.
Btla−/− mice have lower CD8 T effector and memory cell generation. A) WT and Btla−/− mice were infected i.p. with 5×105 CFU Listeria-OVA. Seven days later, splenocytes were isolated and endogenous CD8+ OVA -tetramer+ cells were analyzed by flow cytometry. B) WT and Btla−/−−/− mice were primarily infected i.p. with 1×104 CFU Listeria-OVA. Two months later, mice were re-challenged with 5×106 CFU Listeria-OVA. Seven days later, splenocytes were isolated and endogenous CD8+ OVA -tetramer+ cells and intracellular IFNγ were analyzed by flow cytometry. C) Same as in A, ten and twenty days after infection, splenocytes were isolated and endogenous CD8+ OVA-tetramer+ cells were analyzed by flow cytometry. D) Same as in A and B, three days after infection the bacteria burden in the spleen and liver was tested. The results are representative of five independent experiments, four to five mice in each group and data are represented as mean +/− SEM. See also Figure S2.
Memory T cells are known to play a major role for rapid clearance of Listeria during reinfection. We detected much lower Listeria burden with lower number of memory T cells (Figure 2D). However, the T cell responses in Btla−/− mice is similar after peptide vaccination (Figure S2). Therefore, this data suggests that there might be a completely undiscovered role for the HVEM-BTLA pathway through non-T cells during the host immune responses to Listeria infection.
BTLA inhibits the innate immune response to Listeria infection
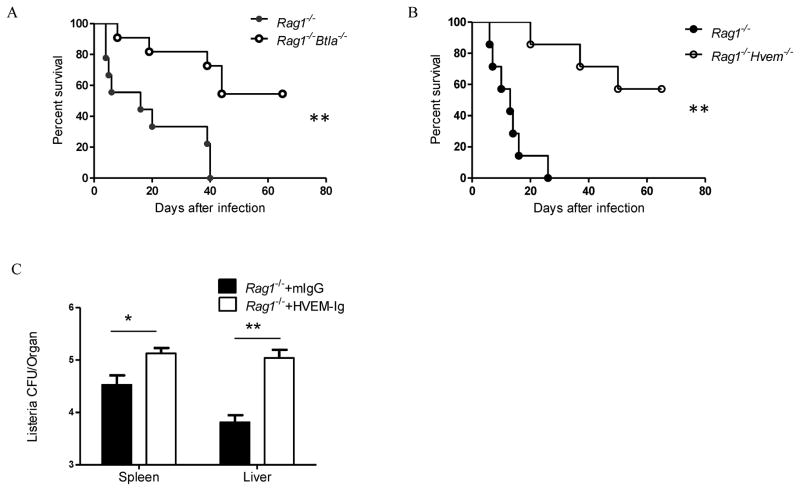
To explore the potential mechanism, we designed several experiments to determine whether BTLA could regulate the innate immune response independently of the adaptive response. To avoid potential feedback from adaptive immune cells, B6 Rag1−/− mice were crossed to Btla−/− and Hvem−/− mice to produce Btla−/−Rag1−/− and Hvem−/−Rag1−/− mice to avoid the influence of adaptive immune system. To directly test whether HVEM and BTLA inhibits the innate immune response, we infected both DKO mice with Listeria and monitored the mice for survival. We found that both Btla−/−Rag1−/− and Hvem−/−Rag1−/− mice survived longer than Rag1−/− controls and with lower bacteria burden (Figure 3A, B and Figure S3A and B), indicating that HVEM and BTLA do inhibit innate immune responses to intracellular Listeria infection.
Figure 3.
BTLA inhibits innate immune response to Listeria infection. A–B) Rag1−/− and Rag1−/− Btla−/−(A) or Rag1−/−Hvem−/− (B) mice were infected i.p. with 1×106 CFU Listeria-OVA and mice survival was observed. C) Rag1−/− mice were infected i.p. with 1×106 CFU Listeria-OVA and treated with 200μg of mouse IgG or HVEM-Ig. Seven days later, the bacteria burden in spleen and liver was tested. The results are representative of three independent experiments, five to seven mice in each group and data are represented as mean +/− SEM. See also Figure S3.
To further determine whether BTLA signaling in innate cells by HVEM interaction is required for the innate response to Listeria infection, we treated Rag1−/− mice with HVEM-Ig to activate BTLA signaling. We did find that Listeria burden was elevated after HVEM-Ig treatment (Figure 3C). Taken together, this data suggests that HVEM and BTLA have an undiscovered role on regulating the innate response to Listeria.
BTLA promotes proliferation of Listeria in CD8α+ DCs for better CD8+ T cell responses
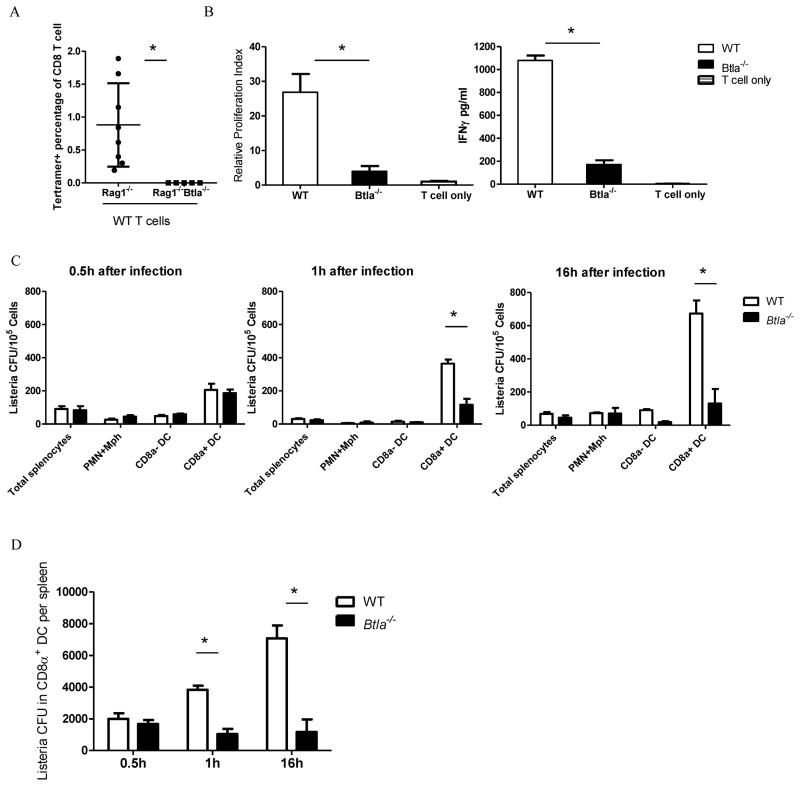
Since BTLA in T cells inhibits T cell activity, this paradigm cannot account for our unexpected finding that the T cell response is reduced in the absence of BTLA in both T and innate cells. Therefore, we hypothesized that BTLA could be regulating non-T cells that help generating the T cell responses, such as the APCs. To test this hypothesis, we adoptively transferred WT splenocytes to Rag1−/− and Btla−/−Rag1−/− mice and infected these mice 14 days later to avoid homeostasis induced activation. In these reconstituted mice, only the expression of BTLA in innate cells were different, not T cells. We compared the bacterial specific T cell responses 7 days after infection and Btla−/−Rag1−/− mice showed dramatically decrease of T cell response compared with Rag1−/− mice (Figure 4A), suggesting that BTLA in innate cells are required for T cell responses. To further define which type of innate cells is responsible for the decreased T cell responses, we isolated DCs, the major APCs for Listerial antigen presentation, from Listeria-OVA infected WT and Btla−/− mice and incubated with naïve OT-I T cells. We found that total DCs from Btla−/− mice have reduced OT-I T cell activation ability (data not shown). Since CD8α+ DCs are the major DC type for priming CTL responses, we further revealed that CD8α+ DCs from Btla−/− mice are less efficient at cross-presenting Listerial antigen to CD8+ T cells resulting in less ability to induce T cell proliferation and IFN-γ production(Figure 4B).
Figure 4.
BTLA promotes proliferation of Listeria in CD8α+ DC s for better CD8+ T cell responses. A) Rag1−/− and Rag1−/− Btla−/− mice were adoptively transferred with 2 × 107 splenocytes. Fourteen days later, mice are infected with Listeria-OVA and OVA specific T cell responses were detect 7 days after infection. B) WT and Btla−/− mice were infected i.v. with 1×107 Listeria-OVA. Sixteen hours after infection, sorted splenic CD8α+ CD11c+ DCs were incubated with purified OVA-specific OT -I T cell. 3H-thymidine was pulsed in the last 18 hours of a three-day culture and cells were harvested for liquid scintillation counting. IFN-γ production in the supernatant was measured on day 2 of the culture. C) WT and Btla−/− mice were infected i.v. with 1×107 Listeria -OVA. Thirty minutes, 1 hour and 16 hours after infection, splenic CD11cloCD8αloCD11b+ granulocyte (PMN) and macrophage (Mφ, including monocytes), CD8α+ and CD8α− CD11c+ DC populations were purified by flow sorting, and sorted populations were lysed and plated onto BHI agar to determine degree of infection. D) Total Listeria CFU in CD8α+ DC per spleen was shown from the same experiment as C). The results are representative of three independent experiments, three to five mice in each group and data are represented as mean +/− SEM. See also Figure S4.
The CD8α+ DC was shown to be early and essential site for replication of for Listeria and replication in these DCs was essential for the induction of anti-Listeria CTL responses (Edelson et al., 2011; Verschoor et al., 2011). Since BTLA deficient CD8α+ DCs express even more MHCII and CD86 after infection (Figure S4A), the lower T cell activation ability is not due to DC activation status, which raises the possibility of lower antigen load in BTLA deficient CD8α+ DCs. Critical to CD8α+ DC function, the platelet was recently shown to be essential for shuttling Listeria from the blood into the CD8α+ DCs (Edelson et al., 2011; Verschoor et al., 2011).
Initially, we asked whether BTLA could affect the trafficking of Listeria from the blood into CD8α+ DCs by means of the platelet. However, the platelet binding ability to Listeria was not altered in Btla−/− mice (Figure S4C). Then we asked whether BTLA could affect the replication of Listeria within CD8α+ DCs, the major infected population of splenocytes, which might further affect the CD8+ T cell activation by impacting the antigen level. In order to detect the proliferation of Listeria in CD8α+ DCs in short time runs, we use intravenous infection instead of previously used intraperitoneal infection. When we checked the Listeria count in CD8α+ DCs 16 hours after infection, we found more than a 5-fold increase in Listeria CFU per CD8α+ DC from WT mice than that from Btla−/− mice (Figure 4C right panel and Figure S4B). There were no difference in Listeria CFU within neutrophils and macrophages. This suggested that BTLA is playing a previously unrecognized role on expanding Listeria inside those specialized DCs.
To determine when and where the Listeria burden was affected through BTLA, we traced the difference to as early as 1 hour after infection (Figure 4C middle panel). Furthermore, there was no difference in Listeria count within CD8α+ DC sooner than 1 hour after infection between WT and Btla−/− mice (Figure 4C left panel). This suggested that there is no defect in pathogen entry into CD8α+ DCs in Btla−/− mice. Not only on a per cell basis was the Listeria burden reduced, but also the total Listeria burden in the entire organ was also reduced in Btla−/− mice (Figure 4D). We observed similar results when HVEM-BTLA interaction was affected in Rag1−/− background or after low dosage infection (data not shown). Therefore, BTLA as the classically inhibitory molecule for T cells could have an opposite role in regulating CD8α+ DCs to promote the pathogen proliferation that dominantly enhances T cell responses, rather than inhibiting T cell responses directly through its ITIMs.
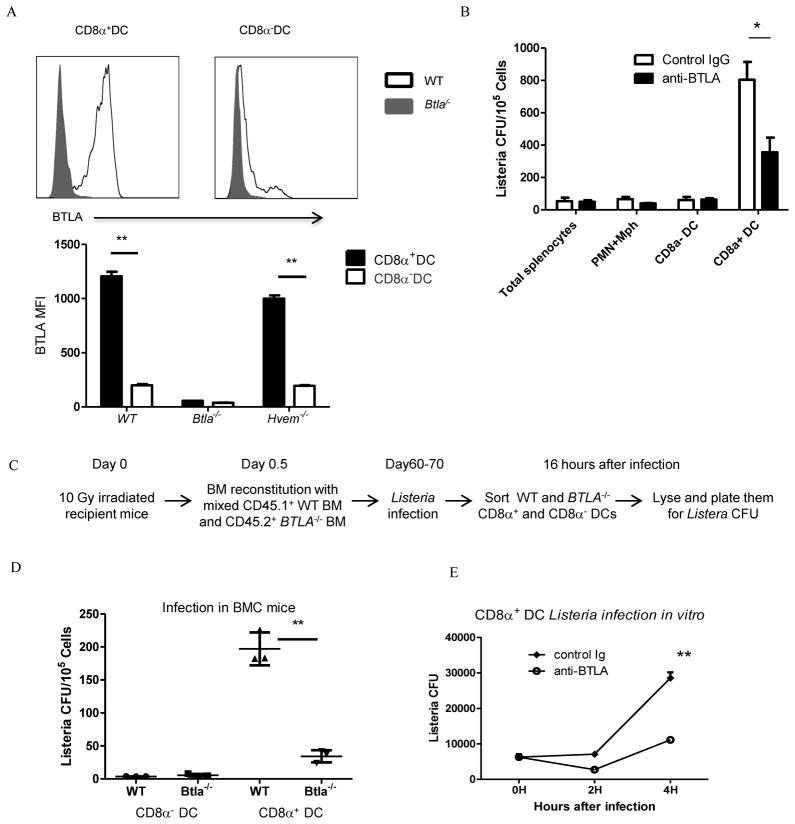
BTLA intrinsically regulates the function of CD8α+ DCs during Listeria infection
Since there is lower Listeria CFU level per CD8α+ DC within 16 hours after infection and there is no reduction of total number of CD8α+ DCs in the spleen of Btla−/− mice (De Trez et al., 2008), BTLA could directly control the function of CD8α+ DCs. To address whether BTLA can directly regulate the CD8α+ DCs, we first asked whether BTLA is selectively expressed in this type of DC. Indeed, BTLA is selectively highly expressed in CD8α+ DCs, but not in other DC subtypes (Figure 5A), which is consistent with previous observation (De Trez et al., 2008). In that study, they focused on the regulatory role of HVEM-BTLA in DC homeostasis. To study whether BTLA signaling in CD8α+ DCs is required for the proliferation of Listeria, we targeted BTLA in WT mice to determine whether functional blockade of BTLA could influence Listeria replication within CD8α+ DCs similarly to the Btla−/− mice. We treated mice with the BTLA blocking mAb (clone 6A6) during infection and found that BTLA signaling blockade indeed affected the replication of Listeria in CD8α+ DCs (Figure 5B). The similar result was observed when mice are treated with another BTLA blocking mAb (clone 6F7, data not shown). These data suggest that the BTLA mediated signaling in CD8α+ DCs is required for functionally regulating the replication of Listeria in these DCs. To further test whether replication of Listeria is through CD8α+ DCs intrinsic mechanism, we constructed mixed bone marrow chimera mice. We transferred CD45.1+ WT and CD45.2+ Btla−/− BM to WT recipient mice (Figure 5C). After infection with Listeria, we sorted out WT and Btla−/− CD8α+ and CD8α− DCs from the same recipient mice and compared the Listeria burden in these cells. We found that there were much less Listeria in Btla−/− CD8α+ DCs than WT CD8α+ DCs (Figure 5D). Furthermore, there is no difference in Listeria burden in CD8α− DCs (Figure 5D). These data suggest that BTLA regulates the function of CD8α+ DCs intrinsically. This conclusion was further proven by the in vitro listeria infection assay in sorted (>98%purity) CD8α+ DCs. Anti-BTLA blocking Ab treatment decreased the proliferation of Listeria in this single sorted cell population. (Figure 5E).
Figure 5.
BTLA directly affects the function of CD8α+ DC s. A) The expression of BTLA and HVEM in CD8α− and CD8α+ DC s from naive WT, Btla−/− and Hvem−/ mice was analyzed by flow cytometry. B) WT mice were treated with Hamster IgG or anti-BTLA (6A6) Ab and infected i.v. with 1×107 Listeria -OVA thirty minutes later. Sixteen hours after infection, splenic CD11cloCD8αloCD11b+ granulocyte (PMN) and macrophage (Mφ, including monocytes), CD8α+ and CD8α− CD11c+ DC populations were purified by flow sorting, and sorted populations were lysed and plated onto BHI agar to determine degree of infection. C) Schematic of the mixed BM chimera experiments. D) WT mice were lethally irradiated (10Gy) and transferred with mix bone marrow (CD45.1 WT and CD45.2 Btla−/− BM). Two months after reconstitution. Mice were infected i.v. with 5×106 Listeria -OVA. Sixteen hours after infection, indicated sorted cell populations were lysed and plated onto BHI agar to determine degree of infection. E) Sorted CD8α+ DCs from WT were infected with Listeria-OVA for 0.5 hour. Free bacteria was removed by Percoll gradient centrifugation. Cell were cultured for another 4 hours in the presence of hamster Ig or anti-BTLA (6A6) Ab. At indicated time, cells were lysed and plated to BHI agar to determine degree of infection. The results are representative of two or three independent experiments, three to five mice in each group and data are represented as mean +/− SEM.
FasL and Fas expression induced by Listeria infection is inhibited by BTLA inCD8α+DC
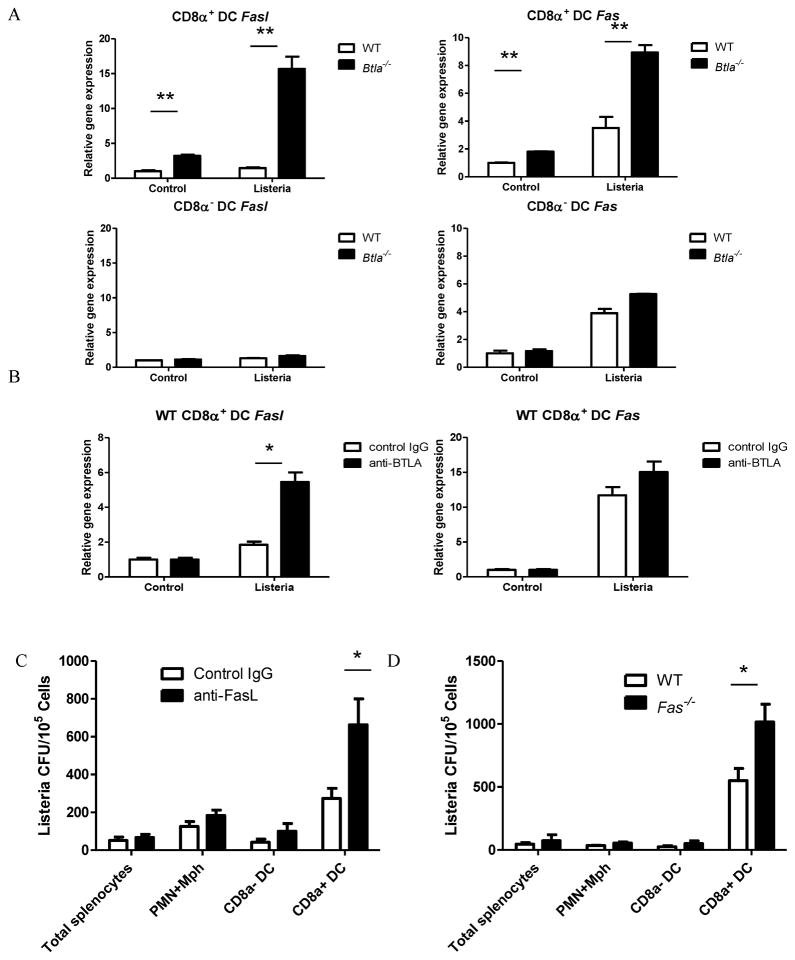
Since our data showed that the BTLA promotes the proliferation of Listeria in CD8α+DC s, we next asked whether BTLA could regulate specific pathways involving CD8α+DC s that might influence intracellular bacterial proliferation. Since BTLA is cloned from apoptotic thymocytes (Han et al., 2004), we wondered whether BTLA could regulate apoptosis-associated molecules, such as TNF and Fas/FasL. We did not see any difference for TNF production (data not shown). Impressively, we have observed that the most prominent alteration was a 7-fold increase in FasL transcripts in CD8α+ DCs from Btla−/− mice compared with WT mice after Listeria infection (Figure 6A). In contrast, no significant changes were observed in mRNA levels of FasL in CD8α− DC s derived from Btla−/− and WT mice (Fig 6A). Since HVEM is the only known binding partner of BTLA, we also compared the FasL and Fas expression in CD8α+DC s from Hvem−/− mice and WT mice. Similarly with Btla−/− mice, CD8α+DC s from Hvem−/− mice showed about 40 times increased expression of FasL after Listeria infection (Figure S5A). Since SHP-1 and SHP-2 are downstream of BTLA signaling for suppressing T cells activation, we wondered whether BTLA uses the same pathway to regulate the expression of FasL. As shown in Figure S5B, inhibiting SHP-1 and SHP-2 by two specific inhibitors could mimic BTLA deficiency induced FasL upregulation during Listeria infection. In this assay, there is no exogenous signal for activating BTLA. We think that the interaction between endogenous HVEM-BTLA is responsible for the activation of BTLA signaling in the steady status.
Figure 6.
BTLA inhibits the expression of FasL and Fas during Listeria infection. A) Sorted splenic CD8α+ and CD8α− DCs from WT and Btla−/− mice were infected with Listeria-OVA in vitro for 6 hours. The expression level of fasL and fas mRNA was analyzed by quantitative real-time PCR. B) Sorted splenic CD8α+ DCs from WT were infected with Listeria-OVA for 6 hours in the presence of hamster Ig or anti-BTLA (6A6) Ab. The expression level of fasL and fas mRNA was analyzed by quantitative real-time PCR. The results are representative of three independent experiments. C) Btla−/− mice were infected i.v. with 1×107 Listeria -OVA and treated with hamster Ig or anti-FasL Ab. Sixteen hours after infection, splenic CD11cloCD8αloCD11b+ granulocyte (PMN) and macrophage (Mφ, including monocytes), CD8α+ and CD8α− CD11c+ DC populations were purified by flow sorting, and sorted populations were lysed and plated onto BHI agar to determine degree of infection. D) WT and Fas−/− mice were infected i.v. with 1×107 Listeria-OVA. Sixteen hours after infection, splenic CD11cloCD8αloCD11b+ granulocyte (PMN) and macrophage (Mφ, including monocytes), CD8α+ and CD8α− CD11c+ DC populations were purified by flow sorting, and sorted populations were lysed and plated onto BHI agar to determine degree of infection. The results are representative of two independent experiments, four mice in each group and data are represented as mean +/-SEM. See also Figure S5.
To specifically address whether the HVEM-BTLA signaling pathway regulates at the functional rather than developmental level, we targeted this pathway in CD8α+ DCs from WT mice using the antagonistic anti-BTLA mAb during Listeria infection. Consistently, the anti-BTLA antibody increased the expression of FasL during Listeria infection (Figure 6B). To further prove that the upregulation of FasL in CD8α+ DCs from Btla−/− mice counts for the lower Listeria proliferation, we blocked the FasL-Fas interaction by a blocking anti-FasL antibody to see whether it would rescue the proliferation of Listeria in BTLA deficient CD8α+ DCs. Indeed, after treatment with anti-FasL antibody, Listeria proliferation was elevated in BTLA deficient CD8α+ DCs (Figure 6C). To determine whether Fas is essential for Listeria expansion inside CD8α+ DCs, Fas−/− mice were infected with Listeria. Indeed, the Listeria level in CD8α+ DCs from Fas−/− mice is elevated compared WT mice (Figure 6D). These data suggest that the HVEM-BTLA interaction is required for the suppression of the expression of FasL and Fas, which is responsible for the lower Listeria proliferation in BTLA deficient CD8α+ DCs.
DISCUSSION
The current dogma is that HVEM-BTLA signaling inhibits T cell function (Krieg et al., 2007; Sedy et al., 2005; Sun et al., 2009; Wang et al., 2005). We have now demonstrated that cross-linking BTLA by HVEM suppresses T cell responses against Listeria, while blocking BTLA enhances both primary and memory T cell responses against the infection. Consistently, we have demonstrated that Btla−/− and Hvem−/− mice exhibited lower Listeria burden than WT mice during the early or memory phase of infection. Therefore, we expected that Listeria-specific CD8+ T cells would be increased in Btla−/− and Hvem−/− mice after Listeria infection. To our surprise, Ag-reactive CD8+ T cells were instead reduced in both KO strains after infection. Intriguingly, the data raised the possibility that the HVEM-BTLA pathway through non-T cells play a dominant role in generation of T cell responses after Listeria infection.
We have further demonstrated that the BTLA pathway controls the function of CD8α+ splenic DCs, thereby increasing the load of intracellular pathogen within the host for improved T cell priming. 1) BTLA signaling can impact the innate immune response in addition to its classically known role on the adaptive immune response: Btla−/−Rag1−/− and Hvem−/−Rag1−/− mice survived longer than the Rag1−/− controls. 2) Direct BTLA signaling controls the function of CD8α+ DCs: The proliferation of Listeria per CD8α+ DC in Btla−/− mice was lower than in WT mice, resulting in less antigenic load for priming the CD8+ T cell response. 3) BTLA in CD8α+ DCs regulated the expression of FasL and Fas in DCs especially after Listeria infection, which could be responsible for the reduced proliferation of Listeria in CD8α+ DCs from Btla−/− mice. Therefore, we propose a role for the HVEM-BTLA pathway. Unexpectedly, the HVEM-BTLA co-inhibitory pathway for T cells regulates APCs to permit early expansion of intracellular pathogens that enhances the sensing of infection by T cells.
Recently, it was reported that lymphotoxin (LT) from the B cell could promote vesicular stomatitis virus (VSV) replication in subcapsular sinus (SCS) macrophages, which produce type I interferons to control lethal VSV central nervous system invasion(Moseman et al., 2012). In this case, SCS macrophages function as a “necessary evil” to permit short-term survival of virus in order to promote long-term immunity of the host. This example and our current study present an overarching strategy used by the host to achieve maximum survival at the cost of early local expansion of pathogens. If we consider that the host more frequently encounters sublethal doses of bacterial pathogens, the host innate response can control these lower dosage infections. By permitting the controlled expansion of pathogen in CD8α+ DCs, the host can better alert CD8+ T cells leading to proper T cell memory responses. Therefore, the host will be better prepared to clear higher dosage infections that would otherwise be lethal in the future. When we infected mice with as low as 10 CFUs of Listeria to test this idea, we discovered that we could still detect the CD8+ T cell responses in WT mice, but not in Btla−/− and Hvem−/− mice (data not shown). This suggested that the host innate response can utilize the HVEM-BTLA pathway to permit Listeria expansion inside a key APC in order to amplify this sublethal signal for priming a stronger adaptive response to protect against future lethal infections. It will be also exciting to determine whether various innate cells can host distinct pathogens for better alerting host or protecting vital host organs to promote overall survival.
More recently, platelets have been shown to acquire Listeria in the bloodstream after intravenous administration of Listeria and are required to rapidly transport the Listeria directly to the splenic CD8α+ DCs (Verschoor et al., 2011). Our data indicates that BTLA deficiency doesn’t impact the platelet-mediated transporting of Listeria from blood to splenic CD8α+ DCs. However, the proliferation of Listeria in CD8α+ DCs is dramatically reduced in Btla−/− mice. Therefore, it raises the possibility that BTLA might be required to permit Listeria expansion within the CD8α+ DCs. It is at early counter-intuitive to imagine why the immune system would evolutionarily preserve this function of BTLA to promote the dissemination of bacteria. We would argue that this same regulation of BTLA in CD8α+ DCs actually promotes a mechanism that generates an optimal CD8+ T cell recall response that is required to protect the host from future lethal infections. Specifically, BTLA signaling permits more Listeria expansion, which can help the antigens to be presented more efficiently, thereby facilitating the generation of stronger effector and memory CD8+ T cell responses.
The Fas pathway has long been considered a critical regulator of T cell homeostasis and T cell apoptosis after activation, as deficiency in the Fas pathway causes dysregulated lympho-proliferation leading to autoimmune diseases (Adachi et al., 1996; Takahashi et al., 1994). This pathway involving Fas/FasL might play an additional role in the homeostasis of innate cells. Our study has now revealed that the BTLA pathway regulates Fas and FasL expression that might be important to control Listeria load in infected CD8α+ DCs. It has been reported that Fas signaling in DCs controls DC accumulation and can curtail autoimmune diseases (Chen et al., 2012; Chen et al., 2006; Stranges et al., 2007). We have observed that that BTLA deficient CD8α+ DCs express more FasL after LPS or CpG stimulation (Data not shown). However, the difference is not as dramatic as Listeria infection, suggesting the impact on TLR signaling may contribute partially to the DC function. Whether the Fas pathway controls the survival of CD8α+ DCs upon Listeria infection remains to be determined. Another possibility is that Fas signaling directly suppresses Listeria proliferation inside these DCs. BTLA is highly expressed by CD8α+ DCs, but not by other DC subtypes, which correlates with the regulatory function of BTLA in these DCs. It will be interesting to further dissect how BTLA signaling regulates the expression of Fas/FasL and whether there are multiple cross-talks happening between the T cell and CD8α+ DC involving BTLA, HVEM and the other binding partners that regulate the innate and adaptive immune responses against intracellular bacterial infections. It would also be interesting to determine whether these interactions happen in cis or trans.
Rather than reduced response, Kaye’s group had previously shown enhanced T cell responses against Listeria due to BTLA deficiency. In that study, T cells from both WT and Btla−/− mice were transferred into the same WT recipient, resulting in BTLA deficiency only in T cells but not in non-T cells such as CD8α+ DCs (Krieg et al., 2007). However, in our current experiments, Btla−/− mice, lacking BTLA in both CD8α+ DCs and T cells, have less bacterial specific T cell response after Listeria infection due to fewer Listeria expansion inside those DCs. Overall, those mice have lower Listeria burden. Together, this indicated a dominant role of BTLA is to enhance listerial expansion in CD8α+ DC that lead to enhance overall T cell response during Listeria infection, whereas the inhibitory function of BTLA in T cells plays a minor role.
Thus, BTLA has dual roles in controlling the host response against intracellular pathogens. Firstly, BTLA in CD8α+ DCs can control antigen load by regulating the function of splenic CD8α+ DCs to determine the sensitivity of subsequent CTL responses. Secondly, BTLA in T cells can modulate the amplitude of T cell activation. Our study provides evidence that a co-inhibitory pathway for T cells could play an opposite role to increase overall T cell responses by permitting pathogen proliferation within specialized DCs. On the other hand, BTLA in T cells might be needed to temper over-reactive T cell responses to reduce tissue injury. Targeting BTLA in different cells could potentially be used therapeutically regulate the complementary innate and adaptive immune responses during different infectious condition. In high dosage lethal infections, blocking BTLA in CD8α+ DCs could reduce initial proliferation of pathogens while blocking BTLA in CD8+ T cells could increase anti-bacterial CTL responses. In low dosage sublethal infections, engaging BTLA to increase antigen load may be more efficient for the host to prime memory responses against lethal infections in the future. It will be exciting to study whether BTLA signal in innate cells is essential for other intracellular pathogen infections and whether other co-inhibitory signals play similar balanced roles for fine tuning the sensitivity and amplitude of T cell responses during pathogen infection.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6J (referred as WT) and Thy1.1 congenic mice were purchased from The Jackson Laboratory. Hvem −/− (Wang et al., 2005) and Btla−/− mice (Han et al., 2004) were generated as previously described. OTI CD8+ TCR transgenic mice (generously provide by William R. Heath, The Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia) were bred with B6/Thy1.1 to obtain OTI/Thy1.1 congenic TCR transgenic mice. All mice were maintained under specific pathogen–free conditions. Animal care and use were in accordance with institutional and NIH protocols and guidelines, and all studies were approved by the Animal Care and Use Committee of the University of Chicago.
Peptide, fusion protein, antibodies and flow cytometry
OVA257–264 (SIINFEKL) peptides were synthesized at the University of Chicago Peptide Core facility. Mouse HVEM-Ig fusion protein (Wang et al., 2005) and blocking anti-BTLA monoclonal antibody (clone 6A6, hamster IgG) (Hurchla et al., 2005) was generated as previously described. Mouse IgG and hamster IgG (Sigma Chemical Co.) were used as controls for HVEM-Ig and 6A6 respectively. iTAg™ MHC Tetramer (SIINFEKL-H-2Kb-PE, referred as OVA-tetramer) was purchased from Beckman Coulter. The other fluorescent-conjugated antibodies were purchased from BD Bioscience or Biolegend. Single cell suspensions of cells were incubated with anti-CD16/32 (anti-FcγIII/II receptor, clone 2.4G2) for 10 minutes and then subsequently stained with conjugated antibodies. Dead cells are excluded by 7-AAD staining. The stained cells were analyzed by flow cytometry using a FACS Caliber or FACS Canto (BD Bioscience). The collected data were analyzed by Flowjo software (Tree Star).
Listeria infection, treatment and determination of CFU
The recombinant Listeria strain Listeria-OVA, which secretes OVA was provided by Chyung-Ru Wang (Northwestern University, Chicago, IL) and was grown in brain–heart infusion broth (Difco Laboratories). For titration of bacterial load in tissues, WT and various knockout mice were infected i.p. with indicated CFU of Listeria-OVA. The bacterial dose was verified by plating dilutions of the inoculum on brain-heart infusion agar plates. To block or engage BTLA pathway, mice were administered with 200 μg of anti-BTLA (6A6) or 200 μg of HVEM-Ig by i.p. injection on the same day but before infection. On indicated day after infection, mice were sacrificed and specimens of spleen and liver were examined for bacterial titers. In brief, organs were homogenized and lysed in sterile water with 0.5% Triton X-100, serial dilutions of homogenates were plated on brain-heart infusion agar plates, and colonies were counted after incubation at 37°C for 24 h.
Monitoring antigen-specific CD8+ T cell in vivo
Pooled splenocytes and lymph node (LN) cells were isolated from OTI/Thy1.1 mice, and labeled with 10 μM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) at 37°C for 30 min. CFSE-labeled cells were adoptively transferred into WT mice at 2×107 cells/mouse by i.v. injection, and the recipients were then infected with 1×105 or 5×105 CFU of Listeria-OVA and treated with anti-BTLA or HVEM-Ig, respectively, as indicated. Three days later, splenocytes were isolated for both surface makers and intracellular IFN-γ staining. Briefly, splenocytes from naïve or Listeria-OVA–infected mice were cultured in 24-well plates at a concentration of 2×106 cells/well in the presence or absence of 1μg/ml OVA257–264 for 5 h. For the last 3 h of stimulation, monensin (final concentration of 10μM) was added to block the secretion of cytokines. Cells were washed and stained for cell surface markers. After fixation with 4% paraformaldehyde and permeabilization with 0.15% saponin, cells were then stained with anti–IFN-γ antibody.
Memory responses after Listeria-OVA infection
WT or various knockout mice were primarily infected i.p. with 1×104 CFU Listeria-OVA. Two months later, mice were re-challenged with 5×106 CFU Listeria-OVA and treated with 200 μg HVEM-Ig or mIgG, Hamster Ig or anti-BTLA (6A6) Ab. Six days later, splenic endogenous CD8+OVA -tetramer+ cells and intracellular IFNγ were analyzed by flow cytometry.
Generation of bone marrow chimeras
WT mice were lethally irradiated with a single dose of 10 Gy. The next day irradiated mice were adoptively transferred i.v. with 1:1 mixed 2×106 WT (CD45.2+) and Btla−/− (CD45.1+) donor bone marrow cells. Mice were maintained on sulfamethoxazole and trimethoprim (Bactrim) antibiotics diluted in drinking water for 4 weeks after reconstitution. Mice were infected with Listeria 60–70 days later.
Analysis of binding between Listeria and platelet
Listeria-OVA was labeled with 5 μM CFSEin PBS for 30 min at 37 °C at a density of 1 × 109 CFU per ml. For analysis of in vivo platelet binding, CFSE-labeled Listeria-OVA were used to infect WT or Btla−/− mice. Blood was collect and immediately diluted 20-fold in flow cytometry buffer (PBS, 0.5% (wt/vol) BSA) containing heparin (50 IU/ml) 1, 5, 60 minutes after infection. Samples were kept at room temperature and manipulated minimally; platelet-specific anti-CD41 (MWReg30; BD Biosciences) was added directly to the sample, followed by dilution of the sample 1:10 in flow cytometry buffer before analysis.
Listeria burden in CD8α+DC
For experiments involving splenic cell sorting, mice were infected intravenously with 1 × 107 CFU Listeria-OVA and spleens were collected after 0.5h, 1h and 16 hours after infection. Spleens were digested with 1mg/ml of collagenase VIII (Sigma Chemical Co.) and 0.2mg/ml of DNase I (Sigma Chemical Co.) at 37°Cfor 20 minutes. Red blood cells were lysed by ACK buffer. Cells were washed once with 5ug/ml gentamicin in PBS for 3 minutes. Cells were enriched by CD11c-PE selection Kit (Stemcell) and Splenic CD11cloCD8αloCD11b+ granulocyte (PMN) and macrophage (Mφ, including monocytes), CD11c+CD8α+ and CD11c +CD8α− populations were purified by flow sorting with FACS AriaII (BD Bioscience). Sorted populations (About 100,000 cells for CD8α+ DC, 200,000 cells for CD8α− DC and 800,000 cells for PMN and macrophage)were lysed and plated onto BHI agar. The degree of infection was calculated by dividing CFU numbers with cell numbers.
FasL and Fas mRNA expression detection
Sorted splenic CD8α+ and CD8α− DC from WT, Btla−/− and Hvem−/− mice were infected with Listeria-OVA in vitro for 6 hours. Total RNA was isolated by RNeasy mini kit (Qiagen) and reverse transcripted to cDNA by Sensiscript RT kit (Qiagen). The expression level of fasL and fas mRNA was analyzed by quantitative real-time PCR. The primers used for the assay are the following:
FasL, 5′-AGAAGAAGGACCACAACACAAATC, 5′-TCACGGAGTTCTGCCAGTTC;
Fas, 5′-TGCGATTCTCCTGGCTGTG, 5′-TTGTATTGCTGGTTGCTGTGC;
β-actin, 5′-ACACCCGCCACCAGTTCGC, 5′-ATGGGGTACTTCAGGGTCAGGATA.
Cytokine analysis
Cytokine production from the culture supernatant was measured by mouse inflammation cytokine beads array kit according to the manufacturer’s protocol (BD Bioscience).
In Vitro T cell stimulation assay by DCs
WT and Btla−/− mice were infected i.v. with 1×107 Listeria-OVA. Sixteen hours after infection, 5 × 104 sorted splenic DCs or CD8α+ CD11c+ DCs were incubated with 2 × 105 purified OT-I T cells for 2 days, pulsed with 1 μCi of 3H-thymidine for 18 hours, and harvested for liquid scintillation counting.
Statistical analysis
Mean values were compared using an unpaired Student’s two-tailed t test. Error bars represent SD. Statistically significant differences p<0.05, and p<0.01 are noted with *, and ** respectively.
Supplementary Material
HIGHLIGHTS.
BTLA suppresses Listeria-specific CD8+ T cell responses
BTLA signaling in CD8α+ DCs promotes Listeria proliferation to enhance CTL
BTLA limits Fas/FasL expression in CD8α+ DCs that inhibits Listeria replication
Acknowledgments
We would like to acknowledge Chyung-Ru Wang (Northwestern University, Chicago, IL) for providing Listeria-OVA. We would like to thank Jonathan Kaye (The Scripps Research Institute, La Jolla, CA) for Btla−/− mice and Kenneth Murphy (Washington University School of Medicine, St. Louis, MO) for anti-BTLA (6A6 and 6F7) antibody. These studies were supported in part by NIH grants DK080736 and CA141975 to YXF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Suematsu S, Suda T, Watanabe D, Fukuyama H, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Enhanced and accelerated lymphoproliferation in Fas-null mice. Proc Natl Acad Sci U S A. 1996;93:2131–2136. doi: 10.1073/pnas.93.5.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldridge JR, Barry RA, Hinrichs DJ. Expression of systemic protection and delayed-type hypersensitivity to Listeria monocytogenes is mediated by different T-cell subsets. Infect Immun. 1990;58:654–658. doi: 10.1128/iai.58.3.654-658.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft GJ, Bosma MJ, Bosma GC, Unanue ER. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell-independent mechanism. J Immunol. 1986;137:4–9. [PubMed] [Google Scholar]
- Berche P, Gaillard JL, Sansonetti PJ. Intracellular growth of Listeria monocytogenes as a prerequisite for in vivo induction of T cell-mediated immunity. J Immunol. 1987;138:2266–2271. [PubMed] [Google Scholar]
- Campisi L, Soudja SM, Cazareth J, Bassand D, Lazzari A, Brau F, Narni-Mancinelli E, Glaichenhaus N, Geissmann F, Lauvau G. Splenic CD8alpha(+) dendritic cells undergo rapid programming by cytosolic bacteria and inflammation to induce protective CD8(+) T-cell memory. Eur J Immunol. 2011;41:1594–1605. doi: 10.1002/eji.201041036. [DOI] [PubMed] [Google Scholar]
- Carrero JA, Calderon B, Unanue ER. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J Exp Med. 2006;203:933–940. doi: 10.1084/jem.20060045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Felix K, Wang J. Critical role for perforin and Fas-dependent killing of dendritic cells in the control of inflammation. Blood. 119:127–136. doi: 10.1182/blood-2011-06-363994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Felix K, Wang J. Critical role for perforin and Fas-dependent killing of dendritic cells in the control of inflammation. Blood. 2012;119:127–136. doi: 10.1182/blood-2011-06-363994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wang YH, Wang Y, Huang L, Sandoval H, Liu YJ, Wang J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- Datta SK, Okamoto S, Hayashi T, Shin SS, Mihajlov I, Fermin A, Guiney DG, Fierer J, Raz E. Vaccination with irradiated Listeria induces protective T cell immunity. Immunity. 2006;25:143–152. doi: 10.1016/j.immuni.2006.05.013. [DOI] [PubMed] [Google Scholar]
- De Trez C, Schneider K, Potter K, Droin N, Fulton J, Norris PS, Ha SW, Fu YX, Murphy T, Murphy KM, et al. The inhibitory HVEM-BTLA pathway counter regulates lymphotoxin receptor signaling to achieve homeostasis of dendritic cells. J Immunol. 2008;180:238–248. doi: 10.4049/jimmunol.180.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussurget O, Pizarro-Cerda J, Cossart P. Molecular determinants of Listeria monocytogenes virulence. Annu Rev Microbiol. 2004;58:587–610. doi: 10.1146/annurev.micro.57.030502.090934. [DOI] [PubMed] [Google Scholar]
- Edelson BT, Bradstreet TR, Hildner K, Carrero JA, Frederick KE, Kc W, Belizaire R, Aoshi T, Schreiber RD, Miller MJ, et al. CD8alpha(+) dendritic cells are an obligate cellular entry point for productive infection by Listeria monocytogenes. Immunity. 2011;35:236–248. doi: 10.1016/j.immuni.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling P, Finger H, Bockemuhl J. Listeria monocytogenes infection in nude mice. Infect Immun. 1975;12:437–439. doi: 10.1128/iai.12.2.437-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Goularte OD, Rufner K, Wilkinson B, Kaye J. An inhibitory Ig superfamily protein expressed by lymphocytes and APCs is also an early marker of thymocyte positive selection. J Immunol. 2004;172:5931–5939. doi: 10.4049/jimmunol.172.10.5931. [DOI] [PubMed] [Google Scholar]
- Harty JT, Schreiber RD, Bevan MJ. CD8 T cells can protect against an intracellular bacterium in an interferon gamma-independent fashion. Proc Natl Acad Sci U S A. 1992;89:11612–11616. doi: 10.1073/pnas.89.23.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- Jensen ER, Glass AA, Clark WR, Wing EJ, Miller JF, Gregory SH. Fas (CD95)-dependent cell-mediated immunity to Listeria monocytogenes. Infect Immun. 1998;66:4143–4150. doi: 10.1128/iai.66.9.4143-4150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Hengartner H, Zinkernagel RM. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur J Immunol. 1994;24:3068–3072. doi: 10.1002/eji.1830241223. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Kaufmann SH, Hug E, Vath U, Muller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985;48:263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg C, Boyman O, Fu YX, Kaye J. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat Immunol. 2007;8:162–171. doi: 10.1038/ni1418. [DOI] [PubMed] [Google Scholar]
- Ladel CH, Flesch IE, Arnoldi J, Kaufmann SH. Studies with MHC-deficient knock-out mice reveal impact of both MHC I- and MHC II-dependent T cell responses on Listeria monocytogenes infection. J Immunol. 1994;153:3116–3122. [PubMed] [Google Scholar]
- Miller ML, Sun Y, Fu YX. Cutting edge: B and T lymphocyte attenuator signaling on NKT cells inhibits cytokine release and tissue injury in early immune responses. J Immunol. 2009;183:32–36. doi: 10.4049/jimmunol.0900690. [DOI] [PubMed] [Google Scholar]
- Mitchell LM, Brzoza-Lewis KL, Henry CJ, Grayson JM, Westcott MM, Hiltbold EM. Distinct responses of splenic dendritic cell subsets to infection with Listeria monocytogenes: maturation phenotype, level of infection, and T cell priming capacity ex vivo. Cell Immunol. 2011;268:79–86. doi: 10.1016/j.cellimm.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittrucker HW, Kohler A, Kaufmann SH. Substantial in vivo proliferation of CD4(+) and CD8(+) T lymphocytes during secondary Listeria monocytogenes infection. Eur J Immunol. 2000;30:1053–1059. doi: 10.1002/(SICI)1521-4141(200004)30:4<1053::AID-IMMU1053>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- Moseman EA, Iannacone M, Bosurgi L, Tonti E, Chevrier N, Tumanov A, Fu YX, Hacohen N, von Andrian UH. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity. 2012;36:415–426. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narni-Mancinelli E, Soudja SM, Crozat K, Dalod M, Gounon P, Geissmann F, Lauvau G. Inflammatory monocytes and neutrophils are licensed to kill during memory responses in vivo. PLoS Pathog. 2011;7:e1002457. doi: 10.1371/journal.ppat.1002457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenhahn M, Kerksiek KM, Nauerth M, Suhre MH, Schiemann M, Gebhardt FE, Stemberger C, Panthel K, Schroder S, Chakraborty T, et al. CD8alpha+ dendritic cells are required for efficient entry of Listeria monocytogenes into the spleen. Immunity. 2006;25:619–630. doi: 10.1016/j.immuni.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Ordway DJ, Orme IM. Listeria monocytogenes infection in beta 2 microglobulin-deficient mice. Infect Immun. 1993;61:1113–1116. doi: 10.1128/iai.61.3.1113-1116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Mateo LR, Chua MM, Weiss SR, Shen H. Perforin-mediated CTL cytolysis counteracts direct cell-cell spread of Listeria monocytogenes. J Immunol. 2002;169:5202–5208. doi: 10.4049/jimmunol.169.9.5202. [DOI] [PubMed] [Google Scholar]
- Sedy JR, Gavrieli M, Potter KG, Hurchla MA, Lindsley RC, Hildner K, Scheu S, Pfeffer K, Ware CF, Murphy TL, Murphy KM. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranges PB, Watson J, Cooper CJ, Choisy-Rossi CM, Stonebraker AC, Beighton RA, Hartig H, Sundberg JP, Servick S, Kaufmann G, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Brown NK, Ruddy MJ, Miller ML, Lee Y, Wang Y, Murphy KM, Pfeffer K, Chen L, Kaye J, Fu YX. B and T lymphocyte attenuator tempers early infection immunity. J Immunol. 2009;183:1946–1951. doi: 10.4049/jimmunol.0801866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Verschoor A, Neuenhahn M, Navarini AA, Graef P, Plaumann A, Seidlmeier A, Nieswandt B, Massberg S, Zinkernagel RM, Hengartner H, Busch DH. A platelet-mediated system for shuttling blood-borne bacteria to CD8alpha+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat Immunol. 2011;12:1194–1201. doi: 10.1038/ni.2140. [DOI] [PubMed] [Google Scholar]
- Wang Y, Subudhi SK, Anders RA, Lo J, Sun Y, Blink S, Wang J, Liu X, Mink K, Degrandi D, et al. The role of herpesvirus entry mediator as a negative regulator of T cell-mediated responses. J Clin Invest. 2005;115:711–717. doi: 10.1172/JCI200522982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.