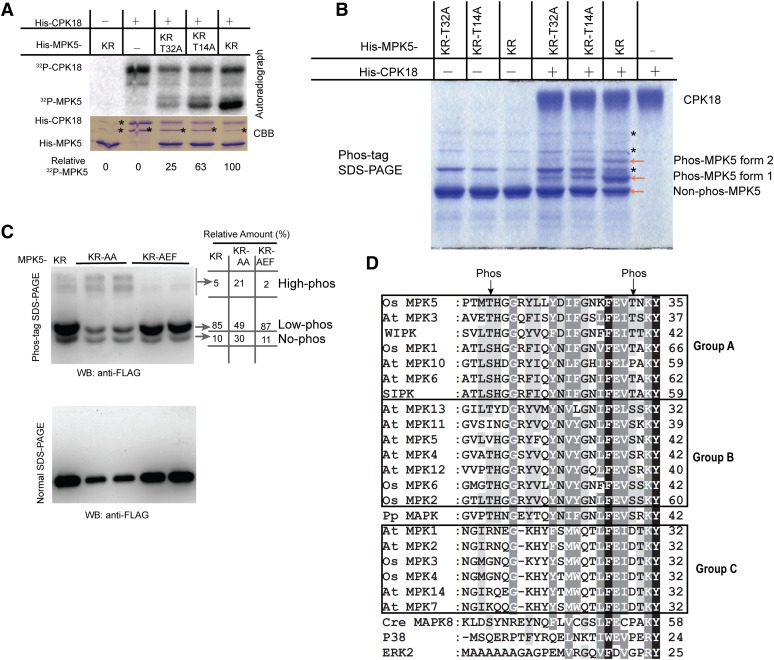
Figure 4.
CPK18 Phosphorylated MPK5 at Thr-14 and Thr-32.
(A) In vitro kinase assay shows that the T14A and T32A mutations drastically impaired MPK5KR phosphorylation by CPK18. The relative phosphorylation level of MPK5 mutants is indicated at the bottom. The asterisks indicate bacterial protein contaminants.
(B) Analysis of MPK5 phosphorylation using a Phos-tag gel. Two phosphorylated forms of MPK5KR (forms 1 and 2) were detected on the Coomassie Brilliant Blue (CBB)–stained Phos-tag gel. The asterisks indicate bacterial protein contaminants.
(C) In vivo phosphorylation profile of MPK5 mutants. FLAG-tagged MPK5KR, MPK5KR-AA (T14A-T32A-K65R), and MPK5KR-AEF (K65R-T194A-Y196F) were expressed in rice protoplasts, and their phosphorylation patterns were analyzed using a Phos-tag gel and immunoblotting. The percentage of differentially phosphorylated forms (no-, low-, and high-phos) was quantified and indicated. WB, immunoblotting.
(D) CPK18 phosphorylation sites (indicated with arrows) are highly conserved in plant group A and B MAPKs but exist neither in group C plant MAPKs nor in MAPKs of humans and C. reinhardtii. N-terminal sequences of all group A/B/C MAPKs from Arabidopsis (At MPK) and rice (Os MPK) were aligned. Two tobacco MAPKs (WIPK and SIPK), human ERK2 and P38, P. patens (Pp) MAPK, and C. reinhardtii (Cre) MAPK8 were also included for comparison. The number at the end of each line indicates the coordinate of the last residue. The high- and medium-level conserved residues among MAPKs are indicated in the alignment with black and gray shading, respectively.
[See online article for color version of this figure.]