Fig. 1.
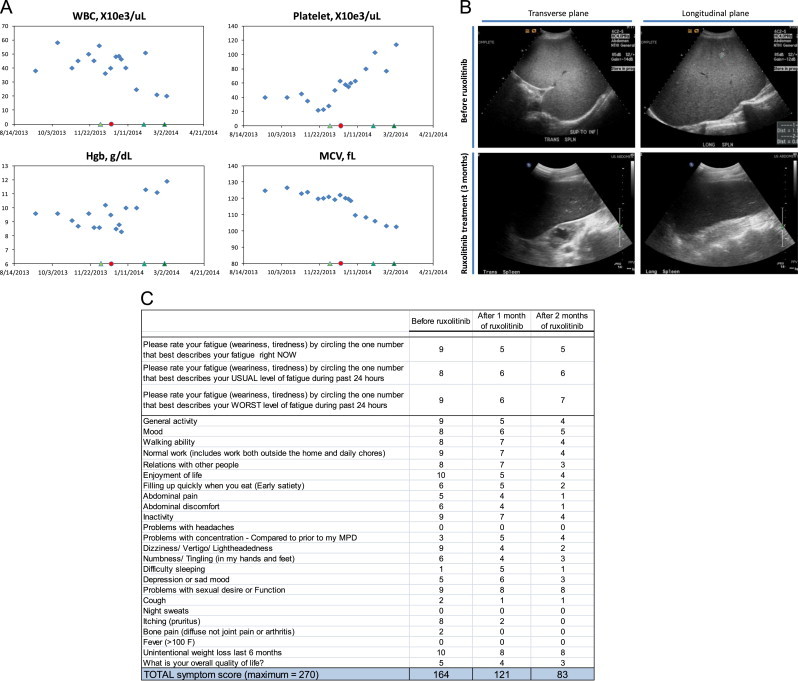
Clinical response in a patient with CSF3R-T618I-positive aCML treated with ruxolitinib. (A) WBC, platelet, Hgb, and MCV laboratory values during ruxolitinib treatment. Refer to case study description for details. Hydroxyurea was stopped at the indicated red circle and ruxolitinib was started at 10 mg twice daily at the indicated green triangle, both on the x-axis. Subsequent green triangles indicate when ruxolitinib was increased to 15 mg twice daily, then to 20 mg twice daily. (B) Spleen volume was determined by conventional prolate ellipsoid method (0.524×W×T×ML; width, thickness, and max length). Two different ultrasound views are shown, the transverse and longitudinal views. Before ruxolitinib treatment, the spleen was diffusely echogenic with hyperechoic nodules. The spleen measured 25.6×19.5×16.6 cm; volume=4342 cm3. After 3 months of ruxolitinib treatment, the spleen was normal in echogenicity and the hyperechoic nodules resolved. The spleen measured 18.5×14.3×7.6 cm; volume=1053 cm3. (C) Shown is the MPN-SAF symptom questionnaire as filled out by the patient. The total symptom score (the sum of each line) is quantitative and a useful tool to monitor symptoms and treatment responses [7]. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
