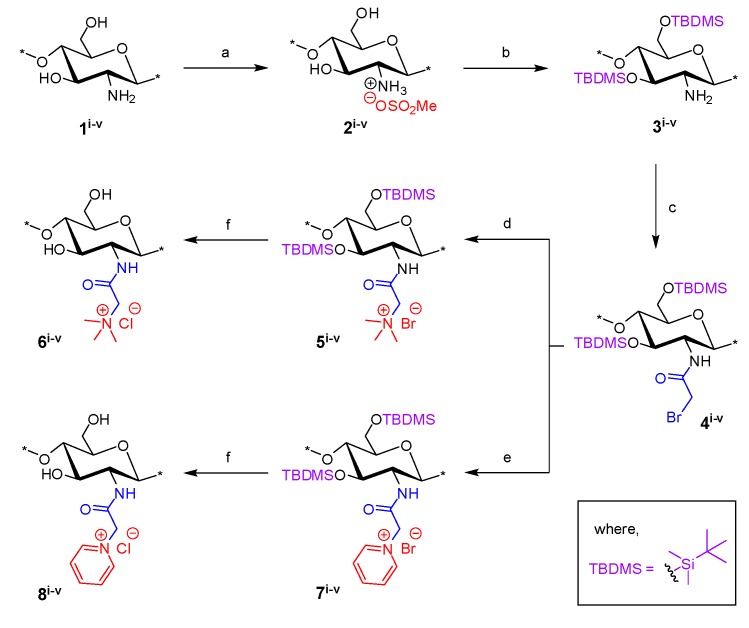
Scheme 1.
Synthesis of final N-(2-(N,N,N-trimethylammoniumyl)acetyl)-chitosan chloride (TMA-CS) (6i–v) and N-(2-(1-pyridiniumyl)acetyl)-chitosan chloride (PyA-CS)(8i–v) derivatives. Reactions and conditions: (a) MeSO3H/H2O (1:1), 10 °C, 1 h (90%); (b) tert-butyl-dimethylsilyl chloride (TBDMSCl), imidazole, DMSO, 25 °C, 24 h (96%); (c) bromoacetyl bromide, Et3N, CH2Cl2, −20 °C, 1 h (92%); (d) Me3N (31%–35% wt in EtOH, 4.2 M),_CH2Cl2,_25 °C, 12 h; (e) pyridine, 25 °C, 24 h; (f) conc HCl/MeOH, 25 °C, 24 h, ion exchanged by (8%) acqueos NaCl (w/v), 1 h, dialysed against de-ionised water, 48h.