Abstract
Detection of chemical signals both in insects and in vertebrates is mediated by soluble proteins, highly concentrated in olfactory organs, which bind semiochemicals and activate, with still largely unknown mechanisms, specific chemoreceptors. The same proteins are often found in structures where pheromones are synthesized and released, where they likely perform a second role in solubilizing and delivering chemical messengers in the environment. A single class of soluble polypeptides, called Odorant-Binding Proteins (OBPs) is known in vertebrates, while two have been identified in insects, OBPs and CSPs (Chemosensory Proteins). Despite their common name, OBPs of vertebrates bear no structural similarity with those of insects. We observed that in arthropods OBPs are strictly limited to insects, while a few members of the CSP family have been found in crustacean and other arthropods, where however, based on their very limited numbers, a function in chemical communication seems unlikely. The question we address in this review is whether another class of soluble proteins may have been adopted by other arthropods to perform the role of OBPs and CSPs in insects. We propose that lipid-transporter proteins of the Niemann-Pick type C2 family could represent likely candidates and report the results of an analysis of their sequences in representative species of different arthropods.
Keywords: odorant-binding proteins, chemosensory proteins, Niemann-Pick type C2 proteins, Insect olfaction, basal hexapods, arthropod chemoreception
Soluble binding proteins in detection and delivery of semiochemicals
Odor detection is accomplished in vertebrates as in insects through a complex and sophisticated sensory system making use of both membrane-bound receptors (Buck and Axel, 1991; Clyne et al., 1999; Vosshall et al., 1999) and soluble binding proteins (Pelosi et al., 1981, 1982; Vogt and Riddiford, 1981). These latter are commonly regarded as solubilizers and carriers of odorants and pheromones, generally hydrophobic compounds, but in recent times evidence has been provided in some insect species for more specific and important roles. In particular, knock-out experiments have demonstrated that an OBP of Drosophila melanogaster, LUSH, is required for olfaction (Xu et al., 2005; Laughlin et al., 2008), while behavior assays with Drosophila mutants (Matsuo et al., 2007; Swarup et al., 2011) and with aphids (Qiao et al., 2009; Sun et al., 2012a) have indicated that OBPs are involved in semiochemical discrimination.
Odorant-binding proteins (OBPs) is the name designating two structurally unrelated families of polypeptides abundantly secreted into the nasal mucus of vertebrates and in the lymph of chemosensilla in insects. OBPs of vertebrates contain 150-160 amino acids (Bignetti et al., 1985; Pevsner et al., 1985; Pelosi, 1994, 1996; Tegoni et al., 2000) and belong to the superfamily of lipocalins (Flower, 1996, 2000), carrier proteins folded in the typical β-barrel shape, with eight β-sheets and one short segment of α-helix close to the C-terminus (Bianchet et al., 1996; Tegoni et al., 1996). OBPs of insects (around 130–140 residues), instead (Vogt and Riddiford, 1981; Pelosi et al., 2006; Vieira and Rozas, 2011; Leal, 2013), are made of six α-helical domains assembled in a compact and stable structure (Sandler et al., 2000; Tegoni et al., 2004). They are characterized by a pattern of six conserved cysteines paired into three interlocked disulfide bridges (Leal et al., 1999; Scaloni et al., 1999).
A third class of soluble binding proteins, named Chemosensory Proteins (CSPs) is also found in olfactory and gustatory organs of insects (McKenna et al., 1994; Pikielny et al., 1994; Angeli et al., 1999; Wanner et al., 2004; Pelosi et al., 2006; Vieira and Rozas, 2011). CSPs are around 100–120 residues long and present a conserved pattern of four cysteines forming two independent loops (Angeli et al., 1999). CSPs are also made of α-helical segments, but assembled in a folding different from that of insect OBPs (Lartigue et al., 2002; Tomaselli et al., 2006; Jansen et al., 2007).
All three classes of soluble proteins contain hydrophobic binding pockets and, despite their structural differences, are believed to perform similar roles in vertebrates and in insects (Pelosi and Maida, 1990; Calvello et al., 2003). Figure 1 reports the three-dimensional folding of a representative protein from each of the three classes.
Figure 1.
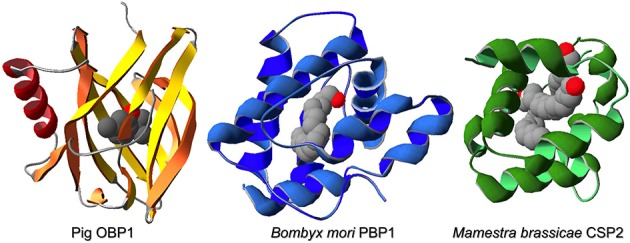
Three-dimensional structures of pig OBP (PDB: 1E06, Vincent et al., 2000), Bombyx mori PBP1 (PDB: 1DQE, Sandler et al., 2000), and Mamestra brassicae CSP2 (PDB: 1N8U, Campanacci et al., 2003), representative examples of a vertebrate OBP, an insect OBP and an insect CSP, respectively. Proteins of these three classes, despite marked structural differences, perform similar roles of transport and solubilization of semiochemicals and are extremely compact and stable. Structures have been visualized using the Swiss-Model PDB Viewer (Guex and Peitsch, 1997).
All three families of proteins, although generally associated with chemodetection, include members expressed outside chemosensory organs.
In vertebrates this phenomenon appeared clear soon after the discovery of the first OBP. In fact, it had already been known for long time that mice and rats excrete small proteins into their urine at concentrations of few milligrams per milliliter (Dinh et al., 1965; Finlayson et al., 1965), but a reasonable explanation for this large waste of energy was not proposed until sequence information was obtained for the bovine OBP, the first to be discovered, which showed high similarity with urinary proteins (Cavaggioni et al., 1987; Cavaggioni and Mucignat-Caretta, 2000). Some proteins were found both in the nasal mucosa of the mouse and in the urine, with the only difference that when secreted into urine they were loaded with species-specific pheromones (Bacchini et al., 1992; Robertson et al., 1993). More examples of mammalian OBPs involved in semiochemical delivery are the boar salivary lipocalins (Marchese et al., 1998; Loebel et al., 2000; Spinelli et al., 2002), the hamster aphrodisin (Singer et al., 1986; Vincent et al., 2001) and the horse sweat lipocalin Equ-c1 (D'Innocenzo et al., 2006). In addition, a lipocalin related to OBPs, the apolipoprotein D, was reported in human sweat, complexed with a volatile fatty acid (Zeng et al., 1996). In all these cases OBPs have been found complexed with pheromones, strongly supporting a function in semiochemical delivery. While it is reasonable to assume that the same or similar proteins might be involved in the dual role of detecting and releasing chemical signals, functions unrelated to chemical communication would appear less obvious. However, if we consider the phenomenon under a wider perspective, we realize that the superfamily of lipocalins, to which vertebrate OBPs belong, includes many different members endowed with diverse functions. In fact, all lipocalins, despite major differences in amino acid sequences, share a conserved architecture (Flower, 1996; Flower et al., 2000). The reason for such versatility of lipocalins is to be found in their extremely stable and compact structure, which allowed adaptation to various uses in different and often challenging conditions.
In insects, a similar phenomenon has only been described in recent years, but several pieces of evidence have been rapidly accumulating in different species. Both OBPs and CSPs have been detected in pheromone glands or in reproductive organs, where they might assist releasing of semiochemicals into the environment. Typical examples are the CSPs found in the pheromone glands of Mamestra brassicae (Jacquin-Joly et al., 2001), Bombyx mori (Dani et al., 2011) and Agrotis ipsilon (Gu et al., 2013), as well as the OBP10 of Helicoverpa armigera and H. assulta (Sun et al., 2012b), the OBP22 of Aedes aegypti (Li et al., 2008) and the CSP91 of Locusta migratoria, produced in the male seminal fluid and transferred to the female, likely with a bound pheromone, during mating (Ban et al., 2013; Zhou et al., 2013). Several OBPs and CSPs are also expressed in the mandibular glands of the honey bee (Iovinella et al., 2011). Other members of both classes seem to be involved in roles completely different from chemical communication. It is noteworthy that the first member of the CSP family, named p10, was discovered in a context not related to chemical communication, as a protein involved in limb regeneration in the cockroach (Nomura et al., 1992; Kitabayashi et al., 1998). Other representative examples of CSPs performing roles unrelated to chemical communication are the CSP5 of the honeybee, only found in ovaries and eggs and required for development of the embryo (Maleszka et al., 2007), and the CSP4 of Helicoverpa armigera and H. assulta, present in high concentration in the lumen of the proboscis and likely acting as a surfactant to help sucking (Liu et al., 2014a). Recently, CSPs have been also indicated as possible scavengers for insecticides, thus providing insects with a sort of resistance to their lethal effects (Liu et al., 2014b; Xuan et al., 2014). Some OBPs have also been related to roles other than chemoreception: OBP56a is expressed in the oral disk of the house fly Phormia regina and has been reported as a fatty acid solubilizer (Ishida et al., 2013). Other examples are two “tandem OBPs” (resulting from two different OBPs joined by a bridge of few amino acids) of the mosquito Aedes aegypti. The first is the salivary protein D7r4, which is involved in antiinflammatory processes (Calvo et al., 2009), the second is the OBP45, reported in the ovaries and eggs with a putative function in reproductive mechanisms responsible for oocyte maturation (Costa-da-Silva et al., 2013; Marinotti et al., 2014).
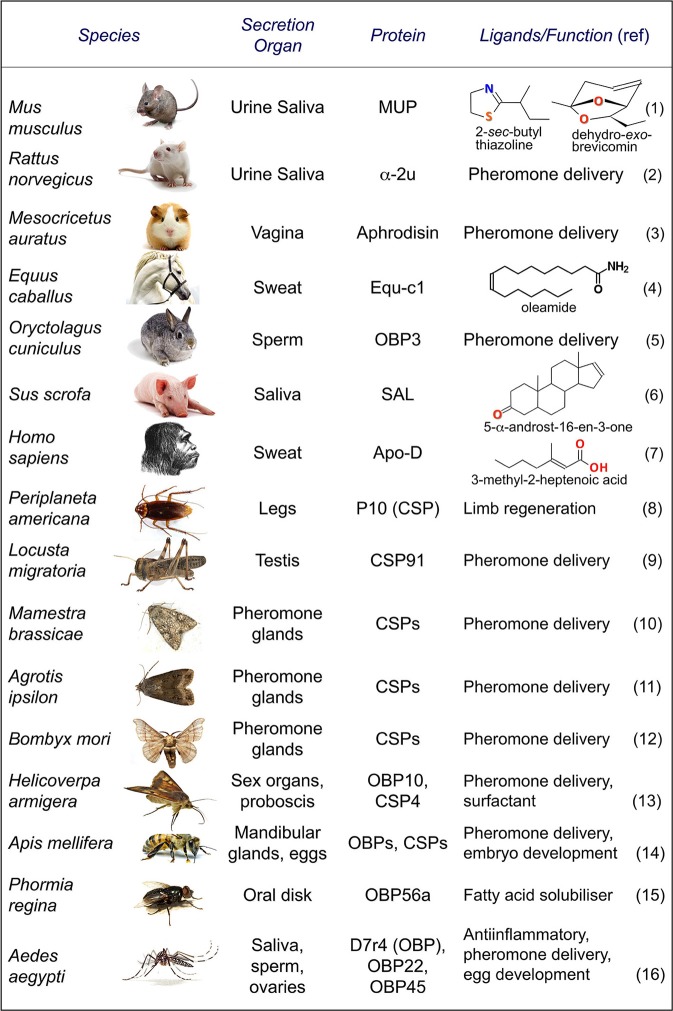
Figure 2 lists representative cases of OBPs (both in vertebrates and in insects) and CSPs utilized for tasks other than chemodetection.
Figure 2.
OBPs and CSPs involved in non-sensory functions. Mammalian OBPs have been found in secretions involved in the delivery of semiochemicals. In several cases, when isolated from such biological fluids, OBPs carry species-specific pheromones. Insect OBPs and CSPs have been reported both in pheromone glands and in reproductive organs, where they likely solubilize and bind specific pheromones. Moreover, members of both classes have been reported in other tissues and shown to be involved in functions unrelated to chemical communication. (1) Finlayson et al., 1965; Bacchini et al., 1992; (2) Dinh et al., 1965; (3) Singer et al., 1986; (4) D'Innocenzo et al., 2006; (5) Mastrogiacomo et al., 2014; (6) Marchese et al., 1998; (7) Zeng et al., 1996; (8) Nomura et al., 1992; Kitabayashi et al., 1998; (9) Zhou et al., 2013; (10) Jacquin-Joly et al., 2001; (11) Gu et al., 2013; (12) Dani et al., 2011; (13) Sun et al., 2012b; Liu et al., 2014a; (14) Iovinella et al., 2011; Maleszka et al., 2007; (15) Ishida et al., 2013; (16) Calvo et al., 2009; Costa-da-Silva et al., 2013; Marinotti et al., 2014; Li et al., 2008.
As observed with lipocalins, compact folding and stability are the characteristics on the basis of such diverse uses of insects OBPs and CSPs, resulting in extreme refractivity to heat, chemicals and proteolytic enzymes (Paolini et al., 1999; Ban et al., 2002; Schwaighofer et al., 2014). Besides, they all present hydrophobic pockets for small and medium size organic chemicals. Therefore, it is reasonable to think that such stable and efficient binding proteins have been utilized for different tasks in various organs, wherever there was need to transport hydrophobic chemicals in aqueous media or to protect some compounds from degradation or else to assure a gradual release of semiochemicals in the environment.
Although such high versatility is associated with all three classes of binding proteins, we can suggest that the structure of insect OBPs is probably the least adaptable to perform different functions, based on the low number of insect OBPs so far reported to perform non-chemosensory functions, with respect to CSPs and vertebrate OBPs. In fact, the folding of insect OBPs is strongly constrained by its three interlocked disulfide bridges, as opposed to more flexible CSPs, which possess two separate bridges, and to vertebrate OBPs, whose β-barrel can swell to a relatively large extent, thus offering diverse structural solutions within a similar folding motif.
An important consequence of this phenomenon for discussing the data that will be presented further on is that sequence similarity alone or the assignment of a new member to one of the three families of binding proteins does not necessarily imply an involvement in chemosensing or in chemical communication.
This review focuses on soluble olfactory proteins of insects with a broader view across all arthropods, in the attempt to outline their possible evolution.
OBPs and CSPs across evolution of insects and arthropods
Given the structural differences between vertebrate OBPs, insects OBPs and CSPs, it is clear that these three families of proteins followed independent evolutionary paths. In particular, it would be of interest to trace down the onset of insect OBPs and CSPs and possibly identify their likely progenitor genes.
So far, insect OBPs have only been described in insects, while some CSPs have been reported in other arthropods (Forêt et al., 2007; Iovinella et al., 2013), although their involvement in chemical communication in non-insect species has not been demonstrated.
Therefore, we have searched for members of these two classes of proteins in basal hexapods (Giribet et al., 2004) and in other arthropods. For those species whose genome has been sequenced we are in the condition of stating whether or not OBPs and CSPs exist and to report the number of their genes. For other species, our search was performed by blasting the EST database using as queries the sequences of species phylogenetically (Giribet and Edgecombe, 2012) as close as possible to those under investigation.
Odorant-binding proteins in insects and arthropods
OBPs have been reported in a large number and variety of insect species (Vieira and Rozas, 2011). The number of their genes in species that had their genome sequenced ranges from a dozen in some ant species (Smith et al., 2011) to more than hundred in some mosquitoes (Manoharan et al., 2013). OBPs are extremely divergent in their sequences and identical amino acids between members of the same species, as well as between species, may be even lower than 10%. The correct assignment of a sequence to the family of OBPs is mainly based on the conserved pattern of six cysteines, determining with their three interlocked bridges the folding and the stability of these proteins. However, OBPs with four cysteines (C-minus OBPs) or with a larger number of such residues (C-plus OBPs) have been reported in many species (Zhou et al., 2004). Based on such criteria and data, the assignment of a new protein to the class of OBPs can be performed with reasonable confidence.
Therefore, using BLAST search tools, we have looked for the presence of OBPs in basal hexapods and in other arthropods. In particular, we have searched protein databases, nucleotide collections and EST sequences in the following groups: Collembola, Diplura, Protura, Archaeognatha, and Zygentoma.
Our search only produced a total of 7 sequences in two species of Collembola, and a single sequence in a Zygentoma species, while we could not detect any gene similar to OBPs in the other groups.
A similar search could not yield any sequence recognizable as OBP in other arthropods, namely Crustacea, Myriapoda, and Chelicerata, as well as in the sister groups Onychophora and Tardigrada. In particular, we used as templates the OBPs of Locusta migratoria, Acyrthosiphon pisum, Bombyx mori, as well as those found in basal hexapods as part of this work. As for some of the species under analysis, such as Daphnia pulex, Ixodes scapularis, and Varroa destructor, partial or complete genome information is available, we can reasonably assume that the class of proteins defined as “insect OBPs” is only found in hexapods. The few OBP genes detected in basal hexapods, such as Collembola and Zygentoma, indicate that OBPs are present since the very first differentiation of the Hexapoda. How these more efficient proteins originated is still unknown, as we were not able to find sequences that might appear as progenitors in other species of arthropods.
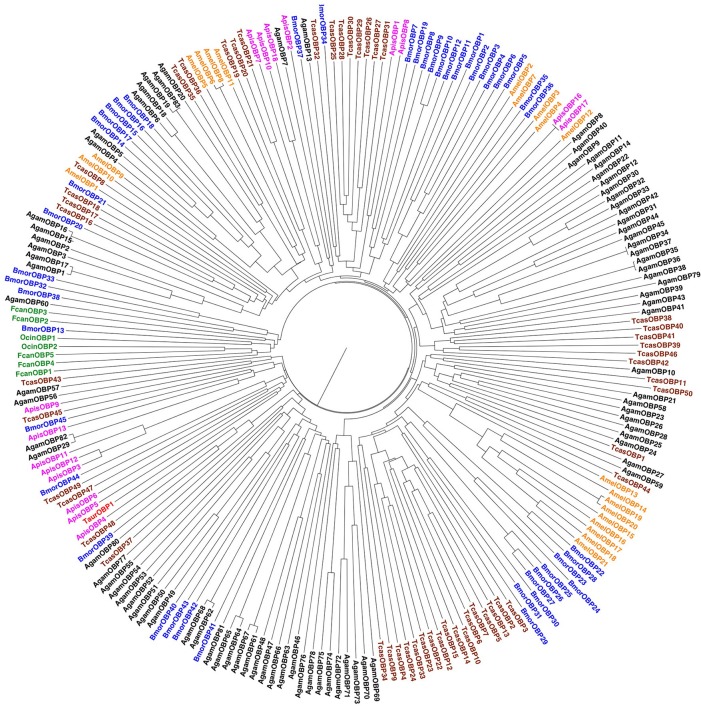
Figure 3 reports a phylogenetic tree built with all the OBPs of selected insect species among those whose genome is available, together with the few members found in Collembola and Zygentoma. More information on the number of OBPs in each species can be found in Table 1. Although the sample of sequences relative to basal hexapods is too small to allow any conclusion, nevertheless we can observe that the five sequences of the collembolan Folsomia candida exhibit a wide divergence, as is the case with other insects OBPs, indicating that their differences can possibly cope with the diversity of semiochemicals in the environment.
Figure 3.
Phylogenetic tree of OBPs from selected species of insects and basal hexapods. Among arthropods, OBPs were only found in Hexapoda. Species and color codes are as follows. Red: Zygentoma (Taur: Thricolepisma aurea); green: Collembola (Fcan: Folsomia candida; Ocin: Orchesella cincta); magenta: Hemiptera (Apis: Acyrthosiphon pisum); brown: Coleoptera (Tcas: Tribolium castaneum); blue: Lepidoptera (Bmor: Bombyx mori); orange: Hymenoptera (Amel: Apis mellifera); black: Diptera (Agam: Anopheles gambiae). Sequences were aligned with the on-line software Clustal-W, using the following parameters. For Pairwise alignment: Protein Weight Matrix: Gonnet; Gap open: 10; Gap extension: 0.1. For Multiple sequence alignment: Protein Weight Matrix: Gonnet; Gap open: 10; Gap extension: 0.2; Gap distance: 5; Clustering: NJ. Phylogenetic trees were visualized with the software Fig Tree (http://tree.bio.ed.ac.uk/software/figtree/). Accession numbers are taken from Vieira and Rozas, 2011.
Table 1.
List of species examined in this work with the number of OBPs, CSPs, and NPC2s so far detected in the databases.
| Taxon | Order | Species | OBP | CSP | NPC2 |
|---|---|---|---|---|---|
| TARDIGRADA | |||||
| Hypsibius dujardini | – | – | 4 | ||
| ONYCHOPHORA | |||||
| Peripatopsis sedgwicki | – | – | 1 | ||
| Epiperipatus sp. | – | – | 1 | ||
| EUCHELICERATA | |||||
| Arachnida | Xiphosura | Limulus polyphemus | – | – | 2 |
| Tetrapulmonata, Araneae | Latrodectus hesperus | – | – | 1 | |
| Loxosceles laeta | – | – | 1 | ||
| Scorpiones | Hottentotta judaicus | – | – | 1 | |
| Acari | Ixodes scapularis | – | 1 | 14 | |
| MYRIAPODA | |||||
| Diplopoda | Julida sp. | – | 2 | – | |
| Archispirostreptus gigas | – | 2 | – | ||
| CRUSTACEA | |||||
| Branchiopoda | Artemia franciscana | – | 1 | 4 | |
| Daphnia pulex | 1 | 12 | |||
| Triops cancriformis | – | 2 | 3 | ||
| HEXAPODA | |||||
| Entognatha | Diplura | Campodea fragilis | – | – | 3 |
| Protura | Acerentomon franzi | – | – | 2 | |
| Collembola | Folsomia candida | 4 | 1 | 4 | |
| Anurida maritima | – | 1 | 6 | ||
| Onychiurus arcticus | – | – | 11 | ||
| Cryptopygus antarcticus | – | 2 | – | ||
| Orchesella cincta | 2 | – | – | ||
| Ectognatha (Insecta) | Archeognatha | Lepismachilis y-signata | – | 2 | – |
| Zygentoma | Thricolepisma aurea | 1 | 1 | – | |
| Orthoptera | Locusta migratoria | 22 | 70 | 2 | |
| Hemiptera | Acyrthosiphon pisum | 16 | 12 | 2 | |
| Coleoptera | Tribolium castaneum | 50 | 19 | 9 | |
| Lepidoptera | Bombyx mori | 45 | 16 | 8 | |
| Hymenoptera | Apis mellifera | 21 | 6 | 5 | |
| Megachile rotundata | 7 | 7 | 4 | ||
| Nasonia vitripennis | 90 | 10 | 5 | ||
| Diptera | Drosophila melanogaster | 52 | 4 | 7 | |
| Culex quinquefasciatus | 109 | 27 | 13 | ||
| Anopheles gambiae | 69 | 8 | 6 | ||
In species where the genome has been published, these figures can be considered as more or less final, in other species their numbers could increase as more information will become available. We have adopted the classification reported in Figure 5 of Giribet and Edgecombe (2012), who suggest that Mandibulata include Myriapoda, Crustacea, and Hexapoda.
Chemosensory proteins in insects and arthropods
Chemosensory proteins are better conserved than OBPs across insect species and can be found in several other arthropods, including Crustacea, Myriapoda, and Euchelicerata. With respect to OBPs, CSPs are in general more widely expressed in different parts of the body, suggestive often of nonspecific functions. As already observed for OBPs, also the number of CSP genes in different species of insects is highly variable, from as few as four in D. melanogaster (Vieira and Rozas, 2011) to at least 70 in L. migratoria (Zhou et al., 2013). Although proteins of both classes, owing to their successful folding and stability, are utilized for different tasks besides chemical communication, it is true that most of the studies on OBPs have been associated with chemoreception, while often CSPs have been reported in connection with other physiological events. The exceptional versatility of CSPs might be related to their high capacity of accepting ligands of different sizes. At least in one case, X-ray crystallography has demonstrated that a CSP can swell to a large extent and bind three molecules of 12-Br-dodecanol (Campanacci et al., 2003). The same adaptability is probably the structural reason why CSPs have been adopted in more than one species as scavengers for insecticides of largely different sizes, such as avermectin (Xuan et al., 2014) and thiametoxam (Liu et al., 2014b).
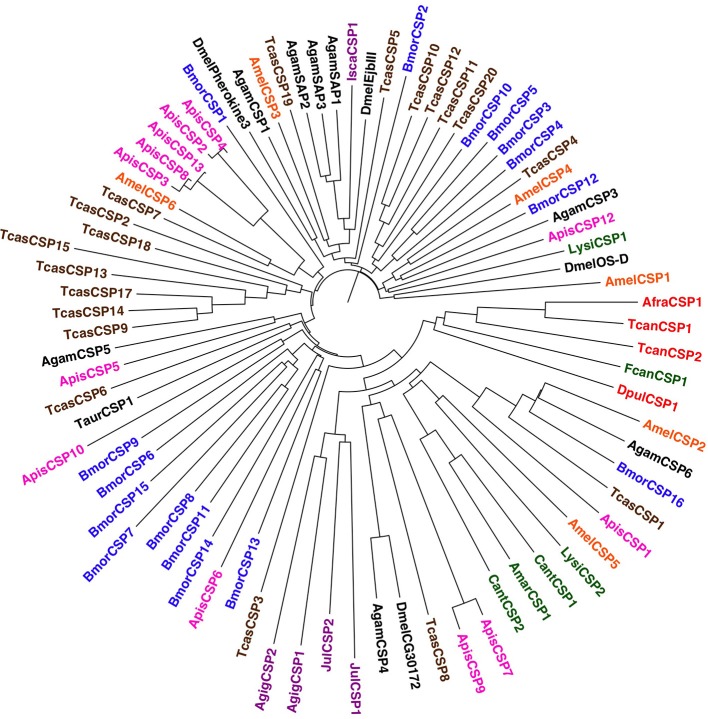
Given such wide repertoire of functions in which CSPs can be involved, it is more difficult to track the use of these proteins in chemodetection across arthropods. To provide a comprehensive picture of CSPs, a phylogenetic tree built on the sequences from selected insect species and all those found so far in other arthropods is reported in Figure 4. More detailed data are provided in Table 1.
Figure 4.
Phylogenetic tree of CSPs from selected species of insects and other arthropods. Apart from Hexapoda, members of the CSP family have also been found in species of Euchelicerata, Myriapoda, and Crustacea. Species and color codes are as follows. Violet: Euchelicerata (Isca: Ixodes scapularis) and Myriapoda (Jul: Julida sp.; Agig: Archispirostrepsus gigas); red: Crustacea (Afra: Artemia franciscana; Dpul: Daphnia pulex; Tcan: Triops cancriformis); green: Collembola (Fcan: Folsomia candida; Amar: Anurida maritima; Cant: Cryptopygus antarcticus; Ocin: Orchesella cincta), Archaeognatha (Lysi: Lepismachilis y-signata) and Zygentoma (Taur: Thricolepisma aurea); magenta: Hemiptera (Apis: Acyrthosiphon pisum); brown: Coleoptera (Tcas: Tribolium castaneum); blue: Lepidoptera (Bmor: Bombyx mori); orange: Hymenoptera (Amel: Apis mellifera); black: Diptera (Dmel: Drosophila melanogaster, Agam: Anopheles gambiae). Sequences were aligned and trees were visualized as in Figure 2. Accession numbers are taken from Vieira and Rozas (2011) or are reported in Table S1.
As a matter of fact, genes encoding CSPs have been found in arthropods other than insects (Pelosi et al., 2006; Zhou et al., 2006), but it would be hard to state that these proteins take the place of OBPs in those species. In fact, no more than one or two sequences have been detected in each species, even when, as in the case of Daphnia pulex, full genomic information is available. It would be more reasonable to link the presence of these CSPs to other functions, such as development. This view is also suggested by the fact that all CSPs of basal hexapods and non-insect arthropods, with the only exception of the single sequence of I. scapularis, cluster in the same large group, together with CSP5 of the honeybee, a protein only found in ovaries and eggs and shown, using experiments of RNA interference, to be required for a correct development of the embryo (Maleszka et al., 2007). Another few insect CSPs, of so far unknown functions, are found in the same branch of the tree; it would be tempting to speculate that perhaps they could also be involved in development or other roles. On the other hand, the single CSP of I. scapularis, so far identified, clusters with three members of the mosquito A. gambiae (SAP1-SAP3) specifically expressed in antennae (Mastrobuoni et al., 2013) and reported to bind several odorants (Iovinella et al., 2013).
Taken together, the information available so far suggests that a role of CSPs in chemodetection, similar to that reported for OBPs, can only be recognized in insects.
Therefore, while insects make use of both OBPs and CSPs in chemical communication, we are left with no candidates for analogous roles in other arthropods.
A new putative class of transport proteins for semiochemicals
To identify suitable candidate proteins in other arthropods which might perform the roles of OBPs and CSPS in Hexapoda chemical communication, we searched among the available databases for other families of binding proteins using the following criteria and guidelines:
there should be a sufficient number of genes in the same species (probably at least a dozen, taking as a reference the 12 OBPs of some ant species), to ensure recognition of complex chemical stimuli using a “combinatorial code” (Malnic et al., 1999);
similarly to OBPs and CSPs, these proteins should be small and soluble;
their structure should include a hydrophobic binding pocket;
being in contact with the external environment, they should be extremely stable to temperature, chemical agents and proteolysis, as much as OBPs and CSPs are.
This last characteristic brings an important consequence which greatly helped our search: small stable proteins are often powerful allergens, as they can reach the blood stream unchanged or slightly affected and trigger immune responses. It is well-known that most lipocalins are allergens, the best examples being β-lactoglobulin (Mäntyjärvi et al., 2000) and several mammalian OBPs, such as Equ-c1, highly abundant in horse sweat (D'Innocenzo et al., 2006). In fact, quite a number of proteins, first reported as allergens, turned out to be members of the mammalian OBP family.
Based on these considerations, we searched for families of allergens presenting a small size and compact structure. We first analyzed the genome of the tick I. scapularis and found 14 sequences classified as Nieman-Pick proteins, type C2 (NPC2), which could fulfill our criteria.
NPC2 in vertebrates
This family of proteins has been widely studied in vertebrates, where they are associated with cholesterol and lipid binding and trafficking (Storch and Xu, 2009). A search through the databases has returned only a single sequence per species of mammals and other vertebrates. Figure 5 reports a phylogenetic tree of NPC2 from representative vertebrates. Another characteristic of NPC2 of vertebrates is their high conservation across species, with identity values higher than 75% between mammalians and around 55–70% between mammals and other vertebrates. This is hardly surprising if their function is the same in all species, mainly to bind cholesterol and lipids.
Figure 5.
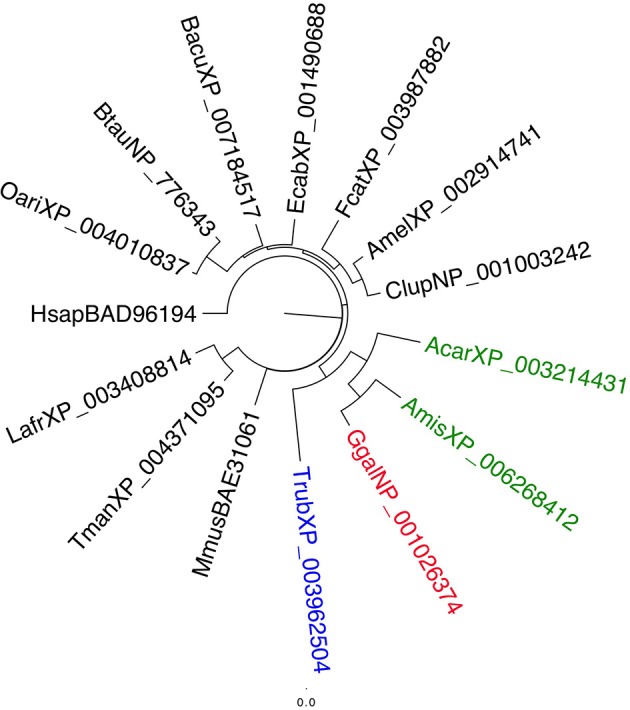
Phylogenetic tree of NPC2 proteins from selected species of vertebrates. These proteins are highly conserved in vertebrates and only a single gene is present in each species. Their role is to bind and transport cholesterol and other lipids. Species and color codes are as follows. Green: Reptiles (Acar: Anolis carolinensis; Amis: Alligator mississippiensis); blue: Fishes (Trub: Takifugu rubripes); red: Birds (Ggal: Gallus gallus); black: Mammals (Mmus: Mus musculus; Fcat: Felis catus; Clup: Canis lupus; Btau: Bos taurus; Ecab: Equus caballus; Oari: Ovis aries; Amel: Ailuropoda melanoleuca; Lafr: Loxodonta africana; Bacu: Balaenoptera acutorostrata; Tman: Trichechus manatus; Hsap: Homo sapiens). Sequences were aligned and trees were visualized as in Figure 2. Names of sequences include accession numbers.
NPC2 in arthropods
During the course of our search, a paper reporting the expression of a member of the NPC2 family in the antennae of the ant Camponotus japonicus (Ishida et al., 2014), supported our hypothesis that such proteins could be involved in chemodetection.
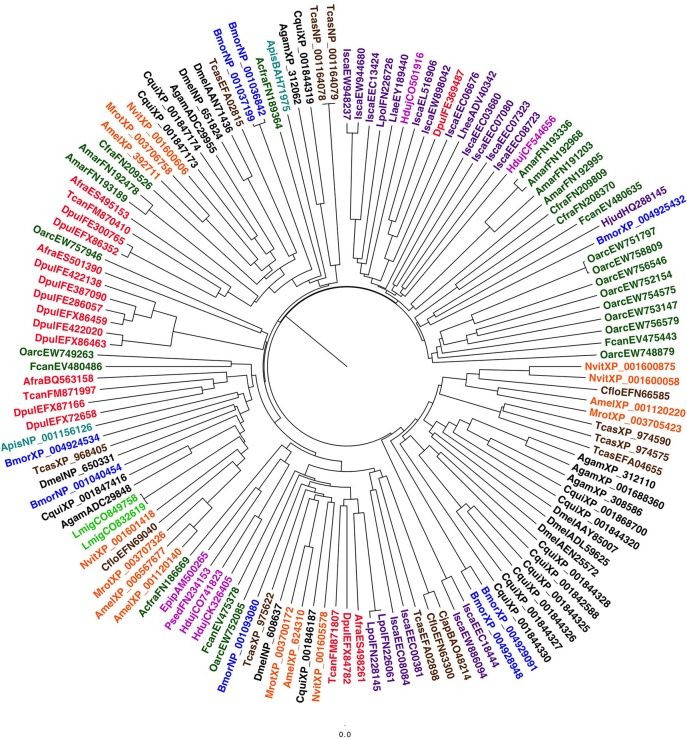
Our analysis through the databases of arthropod proteins and genes, using as a template both our previously found 14 sequences of I. scapularis, as well as that of C. japonicus, returned few genes for each species. Figure 6 reports a phylogenetic tree of the NPC2 sequences found in representative insect species and all of those so far found in other arthropods, including the sister groups Tardigrada and Onychophora. We can observe that the number of these proteins in each species of insects is variable, between 2 and 13 in the species where genome information is available. To verify whether these genes were actually expressed at the protein level, we searched through the results of our previous proteome projects (Dani et al., 2011; Iovinella et al., 2011; Mastrobuoni et al., 2013; Zhou et al., 2013), as well as those published by other groups (Chan et al., 2006, 2011, 2013; Sirot et al., 2008; Baer et al., 2009; Swarup et al., 2011). We found that four of the five genes of A. mellifera are expressed at the protein level, but only two in the antennae (acc. no. XP_624310; XP_001120140). The products of all these four genes have been detected in several different tissues and organs from individuals of queens, drones and workers, most of them not involved in chemical communication. Moreover, we could not find any of the NPC2 proteins in the antennae nor in other examined tissues of B. mori, A. gambiae, L. migratoria, A. aegypti, and D. melanogaster, as far as reported in the above cited papers.
Figure 6.
Similarity tree of Npc2s from selected species of insects and other arthropods. Phylogenetic tree of NPC2 proteins from selected species of insects and other arthropods, as well as “sister groups.” Species and color codes are as follows: magenta: Tardigrada (Hduj: Hypsibius dujardini) and Onychophora (Psed: Peripatopsis sedgwicki; Epip: Epiperipatus sp.); violet: Euchelicerata (Lpol: Limulus polyphemus; Lhesp: Latrodectus hesperus; Llae: Loxosceles laeta; Hjud: Hottentotta judaicus; Isca: Ixodes scapularis); red: Crustacea (Afra: Artemia franciscana; Dpul: Daphnia pulex; Tcan: Triops cancriformis); green: Collembola (Acfra: Acerentomon franzi; Fcan: Folsomia candida; Amar: Anurida maritima; Oarc: Onychiurus arcticus); light green: Orthoptera (Lmig: Locusta migratoria); light blue: Hemiptera (Apis: Acyrthosiphon pisum); brown: Coleoptera (Tcas: Tribolium castaneum; Cjap: Camponotus japonicus; Cflo: Camponotus floridanus); blue: Lepidoptera (Bmor: Bombyx mori); orange: Hymenoptera (Amel: Apis mellifera; Mrot: Megachile rotundata; Nvit: Nasonia vitripennis); black: Diptera (Dmel: Drosophila melanogaster, Cqui: Culex quinquefasciatus; Agam: Anopheles gambiae). Sequences were aligned and trees were visualized as in Figure 2. Names of sequences include accession numbers.
In other arthropods, apart from the 14 sequences of I. scapularis we found 12 genes encoding NPC2 proteins in Daphnia pulex and 11 in the collembolan Onychiurus arcticus, besides fewer members in other basal hexapods (Table 1). Orthologs of these proteins also are present in related species, such as I. ricinus, D. magna and others, but for better clarity we prefer to limit the number of sequences reported in Figure 6 and Table 1 to those of selected species. We also identified 4 sequences in the tardigradan species Hypsibius dujardini, 3 in Limulus polyphemus (Xiphosura) and one in each of two species of Onychophora, Peripatopsis sedgwicki and Epiparipatus sp. As these data are based on very limited sequence information for all these species (except for I. scapularis and D. pulex, whose genome projects have been published), the actual number of NPC2 expressed in each of them could reasonably be higher.
Three-dimensional structure of NPC2
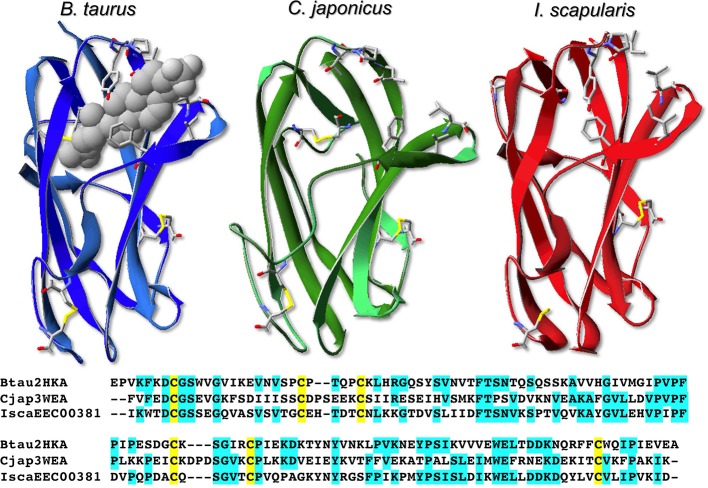
The folding of several NPC2 proteins of vertebrate has been solved. It is a very compact and conserved structure resembling a sort of cylindrical basket made of β-sheets and enclosing the binding cavity for cholesterol and other lipids (Figure 7). It reminds in some way of the β-barrel motif of vertebrate OBPs and more in general of lipocalins. Curiously, NPC2 proteins present a conserved motif of six cysteines paired in three disulfide bridges, similarly to insect OBPs, although this might be no more than a coincidence. Figure 7 also reports a model of one of I. scapularis proteins, built on the structure of the bovine member (PDB ID: 2HKA), together with the structure of C. japonicus NPC2 (PDB ID: 3WEA, Ishida et al., 2014). The entrance to the cavity is gated by a number of amino acids (V59, V64, F66, Y100, P101, I103) conserved or replaced by very similar residues in most of the 14 sequences of I. scapularis. These residues are shown in Figure 7. The binding pocket in the model of the tick protein, as in the structure of the bovine one, is lined with a large number of hydrophobic residues.
Figure 7.
Three-dimensional structures of bovine (PDB ID: 2HKA) and Camponotus japonicus NPC2 (PDB ID: 3WEA), and model of Ixodes scapularis NPC2 (acc. no. EEC00381), built on the bovine NPC2 as a template. The model was obtained using the on-line software “Swiss-Model” (Arnold et al., 2006) and visualized using the Swiss-Model PDB Viewer (Guex and Peitsch, 1997).
Concluding remarks
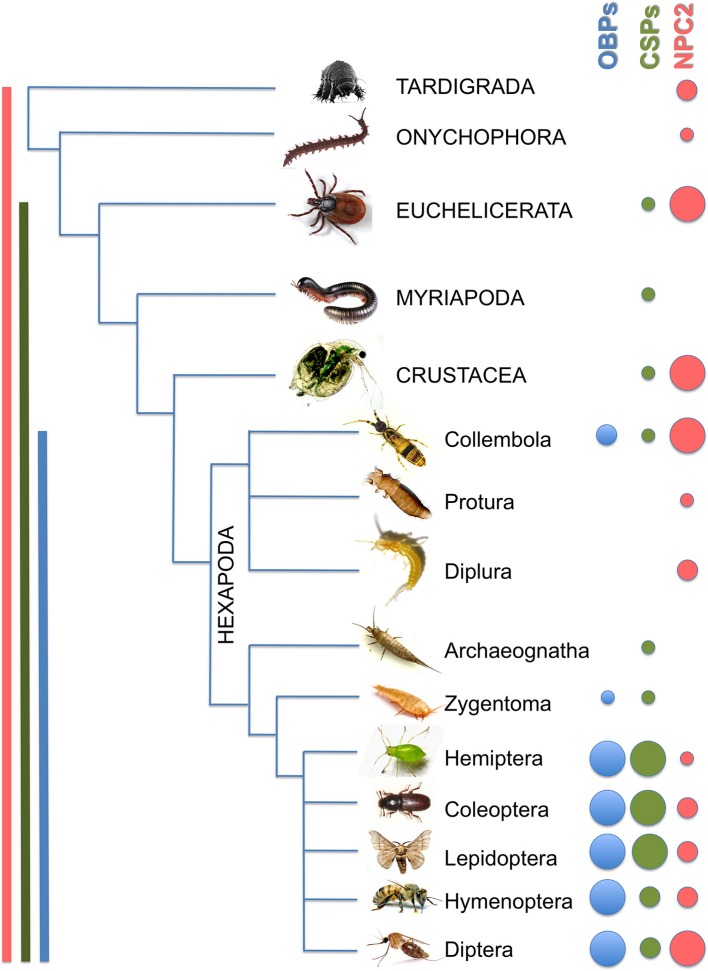
In our analysis of soluble proteins likely to be involved in chemical communication across evolution, as summarized in Figure 8, we have found that:
Figure 8.
Overview of OBPs, CSPs and NPC2 genes in arthropods and sister groups. Taxa are reported in capital letters, Orders in sentence case. Sizes of the dots indicate the maximum number of genes found in each species of the same group (small: 1–2; medium: 3–10; large: >10). Detailed information is reported in Table 1.
OBPs are present in all species of insects so far investigated, including the most primitive ones, but are completely absent in non-insect arthropods.
CSPs are more widely expressed and seem to have appeared earlier than OBPs during evolution. In fact, apart from insects, members of this family have been reported in Crustacea, Myriapoda, and Euchelicerata. However, in these groups the small number of genes in each species does not seem to support a function in chemoreception and other roles could be performed by CSPs in non-insect arthropods.
We propose that in non-insect arthropods proteins of the NPC2 family might fulfill the role of semiochemical carrier performed by OBPs and CSPs in insects. These proteins are small, soluble and secreted. They present a compact folding resembling under certain aspects the β-barrel of vertebrate OBPs with a binding pocket lined by hydrophobic residues.
The relatively large number of NPC2 proteins found in some arthropods and their wide differentiation within the same species suggest that they may have evolved to play a function in binding and discrimination of a variety of semiochemicals.
Certainly the account of soluble proteins of chemoreception in arthropods we have presented in this summary is still fragmentary and incomplete. The fast developing techniques of genome and trascriptome sequencing, as well as proteomic tools, will provide in the near future the necessary information to fill all the gaps and contribute to complete the complex picture of different soluble proteins in chemical communication.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/journal/10.3389/fphys.2014.00320/abstract
References
- Angeli S., Ceron F., Scaloni A., Monti M., Monteforti G., Minnocci A., et al. (1999). Purification, structural characterization, cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria. Eur. J. Biochem. 262, 745–754 10.1046/j.1432-1327.1999.00438.x [DOI] [PubMed] [Google Scholar]
- Arnold K., Bordoli L., Kopp J., Schwede T. (2006). The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22, 195–201 10.1093/bioinformatics/bti770 [DOI] [PubMed] [Google Scholar]
- Bacchini A., Gaetani E., Cavaggioni A. (1992). Pheromone-binding proteins in the mouse Mus musculus. Experientia 48, 419–421 10.1007/BF01923448 [DOI] [PubMed] [Google Scholar]
- Baer B., Heazlewood J. L., Taylor N. L., Eubel H., Millar A. H. (2009). The seminal fluid proteome of the honeybee Apis mellifera. Proteomics 9, 2085–2097 10.1002/pmic.200800708 [DOI] [PubMed] [Google Scholar]
- Ban L., Napolitano E., Serra A., Zhou X., Iovinella I., Pelosi P. (2013). Identification of pheromone-like compounds in male reproductive organs of the oriental locust Locusta migratoria. Biochem. Biophys. Res. Commun. 437, 620–624 10.1016/j.bbrc.2013.07.015 [DOI] [PubMed] [Google Scholar]
- Ban L. P., Zhang L., Yan Y. H., Pelosi P. (2002). Binding properties of a locust's chemosensory protein. Biochem. Biophys. Res. Commun. 293, 50–54 10.1016/S0006-291X(02)00185-7 [DOI] [PubMed] [Google Scholar]
- Bianchet M. A., Bains G., Pelosi P., Pevsner J., Snyder S. H., Monaco H. L., et al. (1996). The three dimensional structure of bovine odorant-binding protein and its mechanism of odor recognition. Nat. Struct. Biol. 3, 934–939 10.1038/nsb1196-934 [DOI] [PubMed] [Google Scholar]
- Bignetti E., Cavaggioni A., Pelosi P., Persaud K. C., Sorbi R. T., Tirindelli R. (1985). Purification and characterization of an odorant binding protein from cow nasal tissue. Eur. J. Biochem. 149, 227–231 10.1111/j.1432-1033.1985.tb08916.x [DOI] [PubMed] [Google Scholar]
- Buck L., Axel R. (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187 10.1016/0092-8674(91)90418-X [DOI] [PubMed] [Google Scholar]
- Calvello M., Guerra N., Brandazza A., D'Ambrosio C., Scaloni A., Dani F. R., et al. (2003). Soluble proteins of chemical communication in the social wasp Polistes dominulus. Cell. Mol. Life Sci. 60, 1933–1943 10.1007/s00018-003-3186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo E., Mans B. J., Ribeiro J. M. C., Andersen J. F. (2009). Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein. Proc. Natl. Acad. Sci. U.S.A. 106, 3728–3733 10.1073/pnas.0813190106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanacci V., Lartigue A., Hallberg B. M., Jones T. A., Giudici-Orticoni M. T., Tegoni M., et al. (2003). Moth chemosensory protein exhibits drastic conformational changes and cooperativity on ligand binding. Proc. Natl. Acad. Sci. U.S.A. 29, 5069–5074 10.1073/pnas.0836654100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaggioni A., Mucignat-Caretta C. (2000). Major urinary proteins, r2u-globulins and aphrodisin. Biochim. Biophys. Acta. 1482, 218–228 10.1016/S0167-4838(00)00149-7 [DOI] [PubMed] [Google Scholar]
- Cavaggioni A., Sorbi R. T., Keen J. N., Pappin D. J., Findlay J. B. C. (1987). Homology between the pyrazine-binding protein from nasal mucosa and major urinary proteins. FEBS Lett. 212, 225–228 10.1016/0014-5793(87)81349-2 [DOI] [PubMed] [Google Scholar]
- Chan Q. W., Chan M. Y., Logan M., Fang Y., Higo H., Foster L. J. (2013). Honeybee protein atlas at organ-level resolution. Genome Res. 23, 1951–1960 10.1101/gr.155994.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Q. W., Howes C. G., Foster L. J. (2006). Quantitative comparison of caste differences in honeybee hemolymph. Mol. Cell. Proteomics 5, 2252–2262 10.1074/mcp.M600197-MCP200 [DOI] [PubMed] [Google Scholar]
- Chan Q. W., Mutti N. S., Foster L. J., Kocher S. D., Amdam G. V., Wolschin F. (2011). The worker honeybee fat body proteome is extensively remodeled preceding a major life-history transition. PLoS ONE 6:e24794 10.1371/journal.pone.0024794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne P. J., Warr C. G., Freeman M. R., Lessing D., Kim J., Carlson J. R. (1999). A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in Drosophila. Neuron 22, 327–338 10.1016/S0896-6273(00)81093-4 [DOI] [PubMed] [Google Scholar]
- Costa-da-Silva A. L., Kojin B. B., Marinotti O., James A. A., Capurro M. L. (2013). Expression and accumulation of the two-domain odorant-binding protein AaegOBP45 in the ovaries of blood-fed Aedes aegypti. Parasit & Vectors 6:364 10.1186/1756-3305-6-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani F. R., Michelucci E., Francese S., Mastrobuoni G., Cappellozza S., La Marca G., et al. (2011). Odorant-binding proteins and Chemosensory proteins in pheromone detection and release in the silkmoth Bombyx mori. Chem. Senses 36, 335–344 10.1093/chemse/bjq137 [DOI] [PubMed] [Google Scholar]
- Dinh B. L., Tremblay A., Dufour D. (1965). Immunochemical study on rat urinary proteins: their relation to serum and kidney proteins (chromatographic separation of the major urinary protein). J. Immunol. 95, 574–582 [PubMed] [Google Scholar]
- D'Innocenzo B., Salzano A. M., D'Ambrosio C., Gazzano A., Niccolini A., Sorce C., et al. (2006). Secretory proteins as potential semiochemical carriers in the horse. Biochemistry 45, 13418–13428 10.1021/bi061409p [DOI] [PubMed] [Google Scholar]
- Finlayson J. S., Asofsky R., Potter M., Runner C. C. (1965). Major urinary protein complex of normal mice: origin. Science 149, 981–982 10.1126/science.149.3687.981 [DOI] [PubMed] [Google Scholar]
- Flower D. R. (1996). The lipocalin protein family: structure and function. Biochem. J. 318, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower D. R. (2000). Experimentally determined lipocalin structures. Biochim. Biophys. Acta 1482, 46–56 10.1016/S0167-4838(00)00147-3 [DOI] [PubMed] [Google Scholar]
- Flower D. R., North A. C., Sansom C. E. (2000). The lipocalin protein family: structural and sequence overview. Biochim. Biophys. Acta 1482, 9–24 10.1016/S0167-4838(00)00148-5 [DOI] [PubMed] [Google Scholar]
- Forêt S., Wanner K. W., Maleszka R. (2007). Chemosensory proteins in the honey bee: Insights from the annotated genome, comparative analyses and expressional profiling. Insect Biochem. Mol. Biol. 37, 19–28 10.1016/j.ibmb.2006.09.009 [DOI] [PubMed] [Google Scholar]
- Giribet G., Edgecombe G. D. (2012). Reevaluating the Arthropod tree of life. Annu. Rev. Entomol. 57, 167–186 10.1146/annurev-ento-120710-100659 [DOI] [PubMed] [Google Scholar]
- Giribet G., Edgecombe G. D., Carpenter J. M., D'Haese C. A., Wheeler W. C. (2004). Is Ellipura monophyletic? A combined analysis of basal hexapod relationships with emphasis on the origin of insects. Org. Divers. Evol. 4, 319–340 10.1016/j.ode.2004.05.001 [DOI] [Google Scholar]
- Gu S.-H., Wu K.-M., Guo Y.-Y., Pickett J. A., Field L. M., Zhou J.-J., et al. (2013). Identification of genes expressed in the sex pheromone gland of the black cutworm Agrotis ipsilon with putative roles in sex pheromone biosynthesis and transport. BMC Genomics 14:636 10.1186/1471-2164-14-636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N., Peitsch M. C. (1997). SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis 18, 2714–2723 10.1002/elps.1150181505 [DOI] [PubMed] [Google Scholar]
- Iovinella I., Bozza F., Caputo B., Della Torre A., Pelosi P. (2013). Ligand-binding study of Anopheles gambiae chemosensory proteins. Chem. Senses 38, 409–419 10.1093/chemse/bjt012 [DOI] [PubMed] [Google Scholar]
- Iovinella I., Dani F. R., Niccolini A., Sagona S., Michelucci E., Gazzano A., et al. (2011). Differential expression of odorant-binding proteins in the mandibular glands of the honey bee according to caste and age. J. Proteome Res. 10, 3439–3449 10.1021/pr2000754 [DOI] [PubMed] [Google Scholar]
- Ishida Y., Ishibashi J., Leal W. S. (2013). Fatty acid solubilizer from the oral disk of the blowfly. PLoS ONE 8:e51779 10.1371/journal.pone.0051779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida Y., Tsuchiya W., Fujii T., Fujimoto Z., Miyazawa M., Ishibashi J., et al. (2014). Niemann-Pick type C2 protein mediating chemical communication in the worker ant. Proc. Natl. Acad. Sci. U.S.A. 111, 3847–3852 10.1073/pnas.1323928111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin-Joly E., Vogt R. G., François M. C., Nagnan-Le Meillour P. (2001). Functional and expression pattern analysis of chemosensory proteins expressed in antennae and pheromonal gland of Mamestra brassicae. Chem. Senses 26, 833–844 10.1093/chemse/26.7.833 [DOI] [PubMed] [Google Scholar]
- Jansen S., Chmelík J., Zídek L., Padrta P., Novák P., Zdráhal Z., et al. (2007). Structure of Bombyx mori chemosensory protein 1 in solution. Arch. Insect. Biochem. Physiol. 66, 135–145 10.1002/arch.20205 [DOI] [PubMed] [Google Scholar]
- Kitabayashi A. N., Arai T., Kubo T., Natori S. (1998). Molecular cloning of cDNA for p10, a novel protein that increases in the regenerating legs of Periplaneta americana (American cockroach). Insect Biochem. Mol. Biol. 28, 785–790 10.1016/S0965-1748(98)00058-7 [DOI] [PubMed] [Google Scholar]
- Lartigue A., Campanacci V., Roussel A., Larsson A. M., Jones T. A., Tegoni M., et al. (2002). X-ray structure and ligand binding study of a moth chemosensory protein. J. Biol. Chem. 277, 32094–32098 10.1074/jbc.M204371200 [DOI] [PubMed] [Google Scholar]
- Laughlin J. D., Ha T. S., Jones D. N. M., Smith D. P. (2008). Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell 133, 1255–1265 10.1016/j.cell.2008.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal W. (2013). Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58, 373–391 10.1146/annurev-ento-120811-153635 [DOI] [PubMed] [Google Scholar]
- Leal W. S., Nikonova L., Peng G. (1999). Disulfide structure of the pheromone binding protein from the silkworm moth, Bombyx mori. FEBS Lett. 464, 85–90 10.1016/S0014-5793(99)01683-X [DOI] [PubMed] [Google Scholar]
- Li S., Picimbon J. F., Ji S. D., Kan Y. C., Qiao C. L., Zhou J. J., et al. (2008). Multiple functions of an odorant-binding protein in the mosquito Aedes aegypti. Biochem. Biophys. Res. Comm. 372, 464–468 10.1016/j.bbrc.2008.05.064 [DOI] [PubMed] [Google Scholar]
- Liu G. X., Xuan N., Chu D., Xie H. Y., Fan Z. X., Bi Y. P., et al. (2014b). Biotype expression and insecticide response of Bemisia tabaci chemosensory protein-1. Arch. Insect Biochem. Physiol. 85, 137–151 10.1002/arch.21148 [DOI] [PubMed] [Google Scholar]
- Liu Y. L., Guo H., Huang L. Q., Pelosi P., Wang C. Z. (2014a). Unique function of a chemosensory protein in the proboscis of two Helicoverpa species. J. Exp. Biol. 217, 1821–1826 10.1242/jeb.102020 [DOI] [PubMed] [Google Scholar]
- Loebel D., Scaloni A., Paolini S., Fini C., Ferrara L., Breer H., et al. (2000). Cloning, post-translational modifications, heterologous expression and ligand-binding of boar salivary lipocalin. Biochem. J. 350, 369–379 10.1042/0264-6021:3500369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleszka J., Forêt S., Saint R., Maleszka R. (2007). RNAi-induced phenotypes suggest a novel role for a chemosensory protein CSP5 in the development of embryonic integument in the honeybee (Apis mellifera). Dev. Genes Evol. 217, 189–196 10.1007/s00427-006-0127-y [DOI] [PubMed] [Google Scholar]
- Malnic B., Hirono J., Sato T., Buck L. B. (1999). Combinatorial receptor codes for odors. Cell 96, 713–723 10.1016/S0092-8674(00)80581-4 [DOI] [PubMed] [Google Scholar]
- Manoharan M., Ng Fuk Chong M., Vaïtinadapoulé A., Frumence E., Sowdhamini R., Offmann B. (2013). Comparative genomics of odorant binding proteins in Anopheles gambiae, Aedes aegypti and Culex quinquefasciatus. Genome Biol. Evol. 5, 163–180 10.1093/gbe/evs131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntyjärvi R., Rautiainen J., Virtanen T. (2000). Lipocalins as allergens. Biochim. Biophys. Acta 1482, 308–317 10.1016/S0167-4838(00)00139-4 [DOI] [PubMed] [Google Scholar]
- Marchese S., Pes D., Scaloni A., Carbone V., Pelosi P. (1998). Lipocalins of boar salivary glands binding odours and pheromones. Eur. J. Biochem. 252, 563–568 10.1046/j.1432-1327.1998.2520563.x [DOI] [PubMed] [Google Scholar]
- Marinotti O., Ngo T., Kojin B. B., Chou S. P., Nguyen B., Juhn J., et al. (2014). Integrated proteomic and transcriptomic analysis of the Aedes aegypti eggshell. BMC Dev. Biol. 14:15 10.1186/1471-213X-14-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrobuoni G., Qiao H., Iovinella I., Sagona S., Niccolini A., Boscaro F., et al. (2013). A proteomic investigation of soluble olfactory proteins in Anopheles gambiae. PLoS ONE 8:e75162 10.1371/journal.pone.0075162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrogiacomo R., D'Ambrosio C., Niccolini A., Serra A., Gazzano A., Scaloni A., et al. (2014). An odorant-binding protein is abundantly expressed in the nose and in the seminal fluid of the rabbit. Plos ONE. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo T., Sugaya S., Yasukawa J., Aigaki T., Fuyama Y. (2007). Odorant-binding proteins OBP57d and OBP57e affect taste perception and host-plant preference in Drosophila sechellia. PLoS Biol. 5:e118 10.1371/journal.pbio.0050118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna M. P., Hekmat-Scafe D. S., Gaines P., Carlson J. R. (1994). Putative Drosophila pheromone-binding proteins expressed in a subregion of the olfactory system. J. Biol. Chem. 269, 16340–16347 [PubMed] [Google Scholar]
- Nomura A., Kawasaki K., Kubo T., Natori S. (1992). Purification and localization of p10, a novel protein that increases in nymphal regenerating legs of Periplaneta americana American cockroach. Int. J. Dev. Biol. 36, 391–398 [PubMed] [Google Scholar]
- Paolini S., Tanfani F., Fini C., Bertoli E., Pelosi P. (1999). Porcine odorant-binding protein: structural stability and ligand affinities measured by fourier-transform infrared spectroscopy and fluorescence spectroscopy. Biochim. Biophys. Acta 1431, 179–188 10.1016/S0167-4838(99)00037-0 [DOI] [PubMed] [Google Scholar]
- Pelosi P. (1994). Odorant-binding proteins. Crit. Rev. Biochem. Mol. Biol. 29, 199–228 10.3109/10409239409086801 [DOI] [PubMed] [Google Scholar]
- Pelosi P. (1996). Perireceptor events in olfaction. J. Neurobiol. 30, 3–19 [DOI] [PubMed] [Google Scholar]
- Pelosi P., Baldaccini N. E., Pisanelli A. M. (1982). Identification of a specific olfactory receptor for 2-isobutyl-3-methoxypyrazine. Biochem. J. 201, 245–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P., Maida R. (1990). Odorant binding proteins in vertebrates and insects: similarities and possible common function. Chem. Senses 15, 205–215 10.1093/chemse/15.2.205 [DOI] [Google Scholar]
- Pelosi P., Pisanelli A. M., Baldaccini N. E., Gagliardo A. (1981). Binding of 3H-2-isobutyl-3-methoxypyrazine to cow olfactory mucosa. Chem. Senses 6, 77–85 10.1093/chemse/6.2.772986147 [DOI] [Google Scholar]
- Pelosi P., Zhou J. J., Ban L. P., Calvello M. (2006). Soluble proteins in insect chemical communication. Cell. Mol. Life Sci. 63, 1658–1676 10.1007/s00018-005-5607-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevsner J., Trifiletti R. R., Strittmatter S. M., Snyder S. H. (1985). Isolation and characterization of an olfactory receptor protein for odorant pyrazines. Proc. Natl. Acad. Sci. U.S.A. 82, 3050–3054 10.1073/pnas.82.9.3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikielny C. W., Hasan G., Rouyer F., Rosbash H. (1994). Members of a family of Drosophila putative odorant-binding proteins are expressed in different subsets of olfactory hairs. Neuron 12, 35–49 10.1016/0896-6273(94)90150-3 [DOI] [PubMed] [Google Scholar]
- Qiao H., Tuccori E., He X., Gazzano A., Field L., Zhou J. J., et al. (2009). Discrimination of alarm pheromone (E)-β-farnesene by aphid odorant-binding proteins. Insect Biochem. Mol. Biol. 39, 414–419 10.1016/j.ibmb.2009.03.004 [DOI] [PubMed] [Google Scholar]
- Robertson D. H., Beynon R. J., Evershed R. P. (1993). Extraction, characterization, and binding analysis of two pheromonally active ligands associated with major urinary protein of house mouse (Mus musculus). J. Chem. Ecol. 19, 1405–1416 10.1007/BF00984885 [DOI] [PubMed] [Google Scholar]
- Sandler B. H., Nikonova L., Leal W. S., Clardy J. (2000). Sexual attraction in the silkworm moth: structure of the pheromone-binding-protein-bombykol complex. Chem. Biol. 7, 143–151 10.1016/S1074-5521(00)00078-8 [DOI] [PubMed] [Google Scholar]
- Scaloni A., Monti M., Angeli S., Pelosi P. (1999). Structural analyses and disulfide-bridge pairing of two odorant-binding proteins from Bombyx mori. Biochem. Biophys. Res. Commun. 266, 386–391 10.1006/bbrc.1999.1791 [DOI] [PubMed] [Google Scholar]
- Schwaighofer A., Pechlaner M., Oostenbrink C., Kotlowski C., Araman C., Mastrogiacomo R., et al. (2014). Insights into structural features determining odorant affinities to honey bee odorant binding protein 14. Biochem. Biophys. Res. Commun. 446, 1042–1046 10.1016/j.bbrc.2014.03.054 [DOI] [PubMed] [Google Scholar]
- Singer A. G., Macrides F., Clancy A. N., Agosta W. C. (1986). Purification and analysis of a proteinaceous aphrodisiac pheromone from hamster vaginal discharge. J Biol. Chem. 261, 13323–13326 [PubMed] [Google Scholar]
- Sirot L. K., Poulson R. L., McKenna M. C., Girnary H., Wolfner M. F., Harrington L. C. (2008). Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochem. Mol. Biol. 38, 176–189 10.1016/j.ibmb.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Zimin A., Holt C., Abouheif E., Benton R., Cash E., et al. (2011). Draft genome of the globally widespread and invasive Argentine ant (Linepithema humile). Proc. Natl. Acad. Sci. U.S.A. 108, 5673–5678 10.1073/pnas.1008617108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S., Vincent F., Pelosi P., Tegoni M., Cambillau C. (2002). Boar salivary lipocalin. Three-dimensional X-ray structure and androsterol/androstenone docking simulations. Eur. J. Biochem. 269, 2449–2456 10.1046/j.1432-1033.2002.02901.x [DOI] [PubMed] [Google Scholar]
- Storch J., Xu Z. (2009). Niemann-Pick C2 (NPC2) and intracellular cholesterol trafficking. Biochim. Biophys. Acta 1791, 671–678 10.1016/j.bbalip.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. F., De Biasio F., Qiao H. L., Iovinella I., Yang S. X., Ling Y., et al. (2012a). Two odorant-binding proteins mediate the behavioural response of aphids to the alarm pheromone (E)-ß-farnesene and structural analogues. PLoS ONE 7:e32759 10.1371/journal.pone.0032759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. L., Huang L. Q., Pelosi P., Wang C. Z. (2012b). Expression in antennae and reproductive organs suggests a dual role of an odorant-binding protein in two sibling Helicoverpa species PLoS ONE 7:e30040 10.1371/journal.pone.0030040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup S., Williams T. I., Anholt R. R. (2011). Functional dissection of Odorant binding protein genes in Drosophila melanogaster. Genes Brain Behav. 10, 648–657 10.1111/j.1601-183X.2011.00704.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegoni M., Campanacci V., Cambillau C. (2004). Structural aspects of sexual attraction and chemical communication in insects. Trends Biochem. Sci. 29, 257–264 10.1016/j.tibs.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Tegoni M., Pelosi P., Vincent F., Spinelli S., Campanacci V., Grolli S., et al. (2000). Mammalian odorant binding proteins. Biochim. Biophys. Acta 1482, 229–240 10.1016/S0167-4838(00)00167-9 [DOI] [PubMed] [Google Scholar]
- Tegoni M., Ramoni R., Bignetti E., Spinelli S., Cambillau C. (1996). Domain swapping creates a third putative combining site in bovine odorant binding protein dimer. Nat. Struct. Biol. 3, 863–867 10.1038/nsb1096-863 [DOI] [PubMed] [Google Scholar]
- Tomaselli S., Crescenzi O., Sanfelice D., Ab E., Wechselberger R., Angeli S., et al. (2006). Solution structure of a chemosensory protein from the desert locust Schistocerca gregaria. Biochemistry 45, 10606–10613 10.1021/bi060998w [DOI] [PubMed] [Google Scholar]
- Vieira F. G., Rozas J. (2011). Comparative genomics of the odorant-binding and chemosensory protein gene families across the arthropoda: origin and evolutionary history of the chemosensory system. Genome Biol. Evol. 3, 476–490 10.1093/gbe/evr033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F., Löbel D., Brown K., Spinelli S., Grote P., Breer H., et al. (2001). Crystal structure of aphrodisin, a sex pheromone from female hamster. J. Mol. Biol. 305, 459–469 10.1006/jmbi.2000.4241 [DOI] [PubMed] [Google Scholar]
- Vincent F., Spinelli S., Ramoni R., Grolli S., Pelosi P., Cambillau C., et al. (2000). Complexes of porcine odorant binding protein with odorant molecules belonging to different chemical classes. J. Mol. Biol. 300, 127–139 10.1006/jmbi.2000.3820 [DOI] [PubMed] [Google Scholar]
- Vogt R. G., Riddiford L. M. (1981). Pheromone binding and inactivation by moth antennae. Nature 293, 161–163 10.1038/293161a0 [DOI] [PubMed] [Google Scholar]
- Vosshall L. B., Amrein H., Morozov P. S., Rzhetsky A., Axel R. (1999). A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 96, 725–736 10.1016/S0092-8674(00)80582-6 [DOI] [PubMed] [Google Scholar]
- Wanner K. W., Willis L. G., Theilmann D. A., Isman M. B., Feng Q., Plettner E. (2004). Analysis of the insect OS-D-like gene family. J. Chem. Ecol. 30, 889–911 10.1023/B:JOEC.0000028457.51147.d4 [DOI] [PubMed] [Google Scholar]
- Xu P., Atkinson R., Jones D. N., Smith D. P. (2005). Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron 45, 193–200 10.1016/j.neuron.2004.12.031 [DOI] [PubMed] [Google Scholar]
- Xuan N., Guo X., Xie H. Y., Lou Q. N., Lu X. B., Liu G. X., et al. (2014). Increased expression of CSP and CYP genes in adult silkworm females exposed to avermectins. Insect Sci. [Epub ahead of print]. 10.1111/1744-7917.12116 [DOI] [PubMed] [Google Scholar]
- Zeng C., Spielman A. I., Vowels B. R., Leyden J. J., Biemann K., Preti G. (1996). A human axillary odorant is carried by apolipoprotein D. Proc. Natl. Acad. Sci. U.S.A. 93, 6626–6630 10.1073/pnas.93.13.6626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J. J., Huang W., Zhang G. A., Pickett J. A., Field L. M. (2004). “Plus-C” odorant-binding protein genes in two Drosophila species and the malaria mosquito Anopheles gambiae. Gene 327, 117–129 10.1016/j.gene.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Zhou J.-J., Kan J., Antoniw J., Pickett J. A., Field L. M. (2006). Genome and EST analyses and expression of a gene family with putative functions in insect chemoreception. Chem. Senses 31, 453–465 10.1093/chemse/bjj050 [DOI] [PubMed] [Google Scholar]
- Zhou X. H., Ban L. P., Iovinella I., Zhao L. J., Gao Q., Felicioli A., et al. (2013). Diversity, abundance and sex-specific expression of chemosensory proteins in the reproductive organs of the locust Locusta migratoria manilensis. Biol. Chem. 394, 43–54 10.1515/hsz-2012-0114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.