Figure 3.
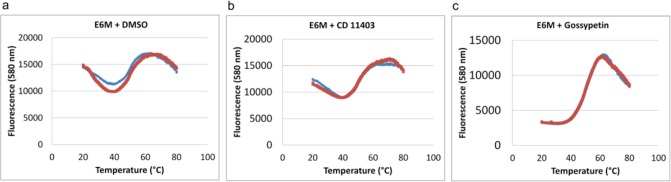
Effect of inhibitors on E6 thermal stability. Replicate data (blue and red) for fluorescence at 580 nm (SYPRO Orange bound to protein) vs temperature for each compound are shown as plots. (a) E6M alone illustrating inherent protein instability as indicated by the initial decrease and fluctuation of the fluorescence signal with increasing temperature over the range of 20–60 °C. (b) E6M with CD11403, representative of a compound that does not significantly stabilize E6M, as there is a modest initial decrease in fluorescence signal (compared to A) with a small but significant increase in fluorescence signal from 20 to 60 °C. (c) E6M with gossypetin, representative of a compound that stabilizes E6M (and presumably directly binds to the protein) as the fluorescence signal is stable between 20 and 37 °C and increases linearly between 37 and 60 °C to give an overall sigmoidal fluorescence signal. This type of thermal denaturation pattern is generally observed for the thermal unfolding of stable proteins.
