Figure 1.
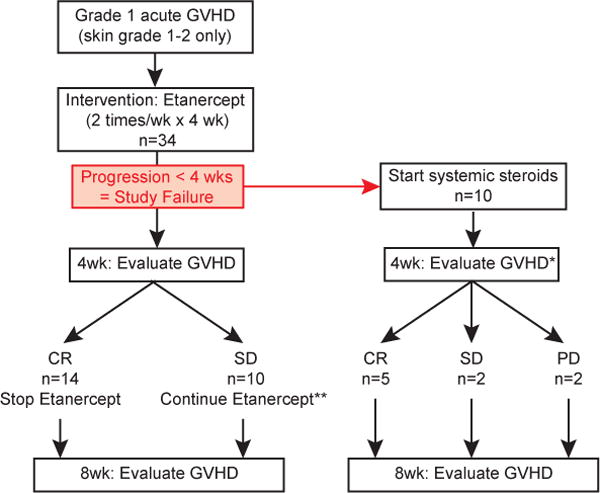
Study schema. Thirty-four patients diagnosed with skin grade 1–2 acute GVHD (overall grade 1) were treated with etanercept twice weekly for four weeks. Patients whose GVHD progressed to grade 2–4 within the first 28 days were considered study failures. In these patients, etanercept therapy was stopped and treatment with systemic steroids was initiated. GVHD was formally evaluated 4 and 8 weeks after diagnosis and onset of etanercept therapy. CR, complete resolution of GVHD; SD, stable disease; PD, progressive disease. *, 1 patient died of idiopathic pneumonia syndrome (IPS) before the 4 week GVHD evaluation; **, 7 of 10 patients with SD at the 4 week evaluation received an additional 4 week course of etanercept treatment.
