Abstract
AIM: To evaluate the efficacy and safety of tolvaptan to treat refractory ascites in decompensated liver cirrhosis patients with or without further complications, such as hepatorenal syndrome and/or hepatocellular carcinoma.
METHODS: Thirty-nine patients (mean age 55 years, males: 32) with decompensated liver cirrhosis and refractory ascites were enrolled. All patients received a combination of tolvaptan (15 mg/d for 5-14 d) and diuretics (40-80 mg/d of furosemide and 80-160 mg/d of spironolactone). The etiology of cirrhosis included hepatitis B (69.2%), hepatitis C (7.7%) and alcohol-induced (23.1%). Changes in the urine excretion volume, abdominal circumference and edema were assessed. The serum sodium levels were also measured, and adverse events were recorded. A follow-up assessment was conducted 1 mo after treatment with tolvaptan.
RESULTS: Tolvaptan increased the mean urine excretion volume (1969.2 ± 355.55 mL vs 3410.3 ± 974.1 mL, P < 0.001), and 89.7% of patients showed improvements in their ascites, 46.2% of whom showed significant improvements. The overall efficacy of tolvaptan in all patients was 89.7%; the efficacies in patients with hepatocellular carcinoma and hepatorenal syndrome were 84.2% and 77.8%, respectively. The incidence of hyponatremia was 53.8%. In patients with hyponatremia, the serum sodium levels increased after tolvaptan treatment (from 128.1 ± 4.22 mEq/L vs 133.1 ± 3.8 mEq/L, P < 0.001). Only mild drug-related adverse events, including thirst and dry mouth, were observed.
CONCLUSION: Tolvaptan is a promising aquaretic for the treatment of refractory ascites in patients with decompensated liver cirrhosis.
Keywords: Tolvaptan, Refractory ascites, Hyponatremia, Decompensation, Liver cirrhosis
Core tip: This study showed that tolvaptan, a new aquaretic drug, is an effective and safe potential treatment for refractory ascites in patients with decompensated liver cirrhosis, especially in patients with further complications, such as hepatorenal syndrome and/or hepatocellular carcinoma. Tolvaptan significantly increased the serum sodium levels in patients with hyponatremia.
INTRODUCTION
Ascites is one of the most common complications of liver cirrhosis[1]. Refractory ascites occur in 15%-20% of all ascites patients and is defined either as an unresponsiveness to restrictions on salt intake and high-dose diuretics, or as a recurrence occurring rapidly within 4 wk post-therapy, according to the American Association for the Study of Liver Diseases (AASLD) guidelines[2]. Refractory ascites are associated with a poor prognosis, and are difficult to treat because of limited treatment options[3-6].
Tolvaptan is a new, oral, selective vasopressin V2-receptor antagonist approved for treating hypervolemic and euvolemic hyponatremia[7,8]. Blockage of V2-receptors by tolvaptan prevents the insertion of aquaporin-2 water channels into the apical cell membrane of the collecting duct, increasing free water excretion without significantly affecting urinary sodium and potassium excretion. As a result, there is reduced water retention with elevated serum sodium levels. The mechanism of action of tolvaptan indicates that it is effective for treating hyponatremia and has a significant role in promoting aquaresis.
Numerous studies have reported the efficacy and safety of tolvaptan for treating ascites and edema in patients with decompensated cirrhosis[8-11]. However, the efficacy and safety of this drug for treating refractory ascites in cirrhotic patients remains unknown. Furthermore, the use of tolvaptan in the subset of patients with complications, such as hepatorenal syndrome and/or hepatocellular carcinoma, has not been explored previously.
Our principal objective was to conduct an observational study to examine the efficacy and safety of tolvaptan to treat refractory ascites and edema in decompensated cirrhotic patients with or without additional complications.
MATERIALS AND METHODS
Study design
A single center, open-label, observational study was conducted in China between May 2012 and July 2013. Patients were recruited between May 2012 and March 2013.
Inclusion criteria
Candidates were selected for study inclusion if they met the criteria for cirrhosis and refractory ascites. A diagnostic work-up for decompensated liver cirrhosis was performed, including assessment of the clinical manifestations, physical examination and laboratory tests. The inclusion criteria were as follows: (1) history of chronic hepatitis and/or signs with various causes; (2) abnormal liver function accompanied by portal hypertension, such as ascites, encephalopathy or esophageal or gastric variceal bleeding; and (3) B-ultrasound scanning (LOGIQ9; GE Company, Fairfield, United States) and four-phase multidetector computed tomography (CT) scan (GE HISPEED DXI; GE Company) results consistent with the signs of liver cirrhosis. Patients with hepatocellular carcinoma were diagnosed using CT or dynamic contrast-enhanced magnetic resonance imaging, in accordance with the diagnostic criteria recommended in the 2010 AASLD guidelines[12]. The definition of refractory ascites underwent a minor revision according to the 2012 AASLD guidelines[2]. Namely, refractory ascites were defined if it was not satisfactorily controlled after a patient had either (1) 1 wk of sodium intake restrictions (< 6 g/d), intermittent albumin infusion (10-20 g per treatment) and high doses of diuretics (more than 160 mg/d of furosemide and 200 mg/d of spironolactone); or (2) 2 wk of therapeutic paracentesis (3000-5000 mL per treatment). Hepatic encephalopathy was assessed in accordance with the guidelines developed by the American Gastroenterological Association[13]. Patients were diagnosed with hepatorenal syndrome in accordance with the International Ascites Club guidelines[14]. Patients were excluded if they had severe cardiovascular, pulmonary, cerebral or hematological complications or severe mental illness.
Ethics
The ethics committee of Beijing You’an Hospital, Capital Medical University approved the current study (protocol number 2011-015) and it was performed in accordance with the ethical standards set forth in the 1964 Declaration of Helsinki and its later amendments. Informed consent was obtained from patients and their families before study participation.
Therapeutic protocol
All patients received oral tolvaptan (15 mg/d for 5-14 d) in addition to a concurrent treatment regimen of sodium intake restrictions (< 6 g/d), intermittent albumin infusion (10-20 g per treatment) and standard diuretic therapy (40-80 mg/d of furosemide and 80-160 mg/d of spironolactone). Patients with abdominal infections were given antibiotics before tolvaptan treatment, and patients with coexisting hepatic encephalopathy also underwent a routine treatment regimen of lactulose. A follow-up assessment was conducted 1-mo post-tolvaptan treatment for all patients.
An electrochemiluminescence immunoassay was used to detect serum markers for hepatitis B virus and hepatitis C virus, in accordance with the manufacturer’s protocol (Roche E170 modular immunoassay analyzer, Roche Diagnostics, Mannheim, Germany). An automatic biochemical analyzer (AU5400, Olympus, Japan) was used to test liver and renal function, including the serum sodium and potassium levels, serum alanine aminotransferase, aspartate aminotransferase, total bilirubin, albumin, creatinine and urea nitrogen.
Efficacy assessment
For ascites, the urine volume was measured over 24 h; we also measured the abdominal circumference and edema of the lower extremities, which were assessed in the morning while the participants had an empty stomach. The overall efficacy of tolvaptan for treating ascites was assessed using a set of three evaluation indicators, which are shown in Table 1.
Table 1.
Definitions for grades representing the measures of improvement for the urine excretion volume, ascites and edema
|
Measure of improvement |
|||
| Significant improvement | Improvement | No improvement | |
| Grade | A | B | C |
| Volume of urine excretion | Increased by > 1000 mL/24 h | Increased by 500-1000 mL/24 h | Increased by < 500 mL/24 h |
| Abdominal circumference | Decreased by > 2 cm | Decreased by 0-2 cm | No change |
| Edema (lower extremities) | A lack of visible pitting | Presence of visible pitting | Distinctly visible pitting |
Survival
The number of patients who survived after 1 mo was recorded to examine the relationship between the short-term correction of hyponatremia and prognostic improvement.
Safety assessment
Patients were monitored throughout the study period, and any occurrences of adverse events and deaths were recorded.
Statistical analysis
Parametric data are expressed as the mean ± standard deviation (mean ± SD) and were assessed using two-tailed t-tests. Levene’s test for equality of variance was conducted concurrently for all t-tests. Categorical data were compared using the Pearson’s χ2 test. The Kaplan-Meier method was used to estimate the 1-mo cumulative patient survival, and between-group differences were tested for significance using the log-rank test. P-values < 0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics software (IBM, version 12.0).
RESULTS
Patient characteristics
Thirty-nine patients who met the study’s eligibility requirements were included. The demographics and other baseline characteristics of these patients are shown in Table 2.
Table 2.
Baseline characteristics of the cirrhotic patients with refractory ascites included in the study n (%)
| Baseline characteristics | Value | |
| Total number of patients | 39 | |
| Age (yr) | 55.0 ± 12.4 | |
| Sex | Male | 32 (82.0) |
| Female | 7 (17.9) | |
| Underlying liver disease | Cirrhosis | 20 (51.3) |
| Cirrhosis with hepatocellular carcinoma | 19 (48.7) | |
| Liver disease etiology | HBV infection | 27 (69.2) |
| HCV infection | 3 (7.69) | |
| Alcoholic cirrhosis | 9 (23.1) | |
| Coexisting hepatorenal syndrome | Type 1 | 2 (5.13) |
| Type 2 | 7 (17.9) | |
| Coexisting hepatic encephalopathy | 13 (33.3) | |
| Child-Pugh score | Class C | 39 (11.8 ± 1.5) |
| MELD score | 39 (37.5 ± 5.6) | |
| Coexisting diabetes | 9 (23.1) | |
| Hyponatremia | Yes | 21 (53.8) |
| No | 18 (46.2) | |
Data are expressed as absolute numbers (percentage) or mean ± SD. HBV: Hepatitis B virus; HCV: Hepatitis C virus; MELD: Model for end-stage liver disease.
Ascites and edema
The administration of tolvaptan resulted in a significant increase in the mean urine excretion volume, from 1969.2 ± 355.55 mL pre-treatment to 3410.3 ± 974.1 mL post-treatment [t(48) = -8.679, P < 0.001; n = 39]. Levene’s test indicated that there were unequal variances in the data sets (F = 27.115, P < 0.001); as a result, the degrees of freedom were adjusted from 76 to 48. The combination of tolvaptan with diuretics effectively increased the urine output in 89.7% of patients with refractory ascites (Table 3). The abdominal circumference was reduced in 82% of patients, and edema was also improved in 91.7% of patients (Table 3).
Table 3.
Summary of the improvement in the urine excretion, ascites and edema after tolvaptan treatment, according to grading criteria, and in the overall improvement n (%)
| n | Significant improvement | Improvement | No improvement | |
| Urine excretion | 39 | 26 (66.7) | 9 (23.1) | 4 (10.3) |
| Abdominal circumference | 39 | 19 (48.7) | 13 (33.3) | 7 (17.9) |
| Edema (lower extremities) | 24 | 17 (70.8) | 5 (20.8) | 2 (8.3) |
| Overall improvement for all patients1 | 39 | 18 (46.2) | 17 (43.6) | 4 (10.2) |
| Overall improvement in patients with coexisting hepatocellular carcinoma1 | 19 | 9 (47.4) | 7 (36.8) | 3 (15.8) |
| Overall improvement in patients with coexisting hepatorenal syndrome (Type 1)1 | 2 | 0 (0) | 0 (0) | 2 (100.0) |
| Overall improvement in patients with coexisting hepatorenal syndrome (Type 2)1 | 7 | 2 (28.6) | 5 (71.4) | 0 (0) |
Overall improvement definitions: Significant improvement = grade of A for urine excretion, ascites and edema; Improvement = grade of B for urine excretion and a grade of improvement for ascites or edema; No improvement = grade of C for urine excretion.
The overall efficacy of tolvaptan was 89.7% (n = 35) in all patients, and 46.2% (n = 18) of these patients had significant improvement (Table 3 and Figure 1). Subgroup analyses indicated that the overall efficacy of tolvaptan in patients with coexisting hepatocellular carcinoma was 84.2% (n = 19) and that the efficacy for patients with coexisting hepatorenal syndrome was 77.8% (n = 9; Table 3 and Figure 1). Tolvaptan was not effective to treat refractory ascites in patients with coexisting Type 1 hepatorenal syndrome.
Figure 1.
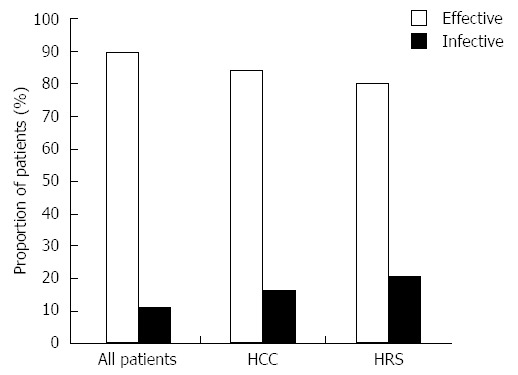
Overall efficacy of 15 mg/d tolvaptan. Overall efficacy of 15 mg/d tolvaptan for 5-14 d in all patients as well as in subgroups of patients with coexisting hepatocellular carcinoma (HCC) and hepatorenal syndrome (HRS) post-tolvaptan treatment.
Hyponatremia
The incidence of hyponatremia (defined as a serum sodium concentration < 135 mEq/L) was 53.8% (21 of 39 patients) in cirrhotic patients with refractory ascites. Tolvaptan caused a significant increase in the serum sodium concentration in patients with hyponatremia (from 128.1 ± 4.22 to 133.1 ± 3.8 mEq/L; Figure 2). There was no significant change in the serum sodium concentration for patients lacking hyponatremia after tolvaptan treatment (137.8 ± 3.02 mEq/L before treatment and 136.9 ± 3.18 mEq/L after treatment; Figure 2). There was no significant relationship between the short-term correction of hyponatremia and the 1-mo patient survival rate [Figure 3; χ2 (2, n = 39) = 0.454, P > 0.05].
Figure 2.
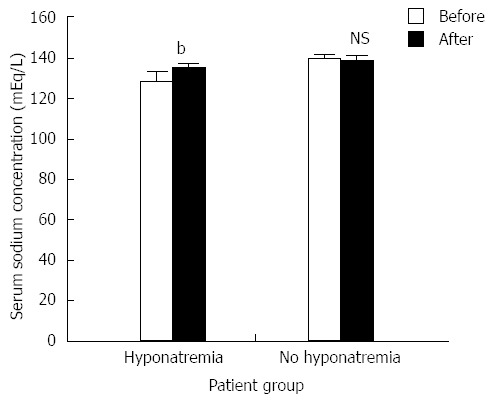
The effects of tolvaptan on serum sodium (mean ± SE) in patients with and without hyponatremia (n = 21 and n = 18, respectively). Tolvaptan (15 mg/d, 5-14 d) treatment (black column) significantly increased serum sodium concentration [t (40) = -4.029, bP < 0.01 vs before treatment group] in patients with hyponatremia, but not in patients without hyponatremia [t (32) = 1.545, P > 0.05 (NS)]. NS: Not significant.
Figure 3.
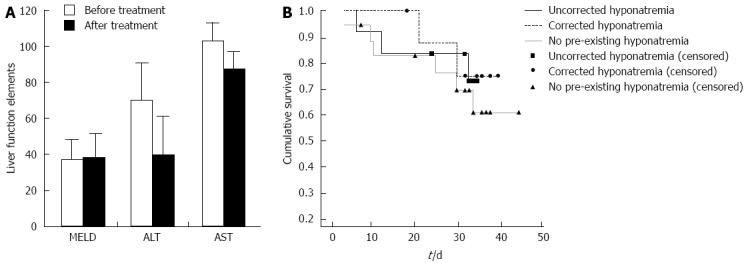
Tolvaptan does not affective liver function. A: Tolvaptan (15 mg/d for 5-14 d) does not affective liver function [model for end-stage liver disease (MELD) score, alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] in patients with preexisting liver cirrhosis (P > 0.05); B: Kaplan-Meier analysis of the 1-mo survival of patients without hyponatremia, as well as with and without short-term correction of hyponatremia, did not show any significant difference between patient groups.
Adverse events
Mild adverse events (thirst and dry mouth) associated with tolvaptan treatment were reported in four and two patients, respectively. No other drug-related adverse events or liver function abnormalities were observed in this study. Improvements were observed in 11 of 13 patients who had coexisting hepatic encephalopathy. In patients with hepatorenal syndrome, five of seven patients with Type 2 syndrome showed improvements during treatment, while two patients with coexisting Type 1 syndrome experienced continuous deterioration of their renal function.
DISCUSSION
The current theory for the development of ascites is complex and has recently evolved to include previously proposed mechanisms[5]. However, to fully understand how tolvaptan affects refractory ascites, the pathogenesis of ascites needs to be briefly explored.
The formation of ascites involves an increase in the hepatic portal vein pressure, which may cause systemic arterial vasodilation, resulting in a decrease in the effective blood volume[15-17]. This decrease in blood volume triggers the activation of the sympathetic nervous system and the renin-angiotensin system; the resultant cascade of events causes the release of arginine vasopressin (AVP, which is alternatively termed antidiuretic hormone). AVP plays a critical role in water reabsorption in the renal collecting ducts[5,8]. However, as mentioned previously, the treatments for refractory ascites are limited. A diet of controlled salt intake, adequate diuretics treatment, large-volume paracentesis, transjugular intrahepatic portosystemic shunting and liver transplantation are the current clinical recommendations[3,18] However, targeting the AVP mechanism presents a potential novel treatment approach for refractory ascites.
Tolvaptan improves ascites and edema in patients with decompensated liver disease, but its efficacy has not been explored previously in the subset of patients with refractory ascites. In the current study, the combination of tolvaptan with diuretics was effective in increasing urine output, decreasing abdominal circumference and reducing edema in patients with refractory ascites. Tolvaptan was effectively treated a substantial proportion of all patients, and 46.2% of the responders experienced significant improvement. Therefore, this study shows that the combination of tolvaptan with diuretics is more effective than standard options for treating refractory ascites in decompensated cirrhotic patients with or without further complications, such an hepatocellular carcinoma and/or hepatorenal syndrome.
A number of studies have demonstrated the efficacy of tolvaptan for treating hyponatremia[19-23]. In this study, tolvaptan significantly increased the serum sodium levels of patients with hyponatremia, while no significant difference was observed for patients lacking hyponatremia, supporting the efficacy of this drug in end-stage cirrhotic patients with refractory ascites and hyponatremia. A 1-mo follow-up assessment revealed that our treatment regimen corrected hyponatremia in 9 of 21 patients (42.9%). This observation is supported by reports from Berl et al[19]. While the relationship between the short-term correction of hyponatremia and survival was not statistically significant, this study had a small sample size. As a result, further studies are warranted to validate this finding. Further investigations are also warranted to definitively assess the clinical use of tolvaptan for treating refractory ascites in patients with decompensated liver cirrhosis.
In January 2013, the US FDA issued a warning for tolvaptan use because of the potential risks of liver injury that were identified during a clinical trial of tolvaptan to treat autosomal dominant polycystic kidney disease. The study found that 3 of 1445 cases treated with tolvaptan had significantly higher serum bilirubin and ALT. However, the clinical trial tolvaptan dose (120 mg/d for 3 years) was significantly higher, and the administration duration was longer than the recommended dosing strategy (15-60 mg/d for 7-30 d) for treating hyponatremia or ascites[24-26]. The United States, Japan and Europe are the main regions using tolvaptan in the clinic. Currently, there are only 311 cases of self-reported adverse events, and there are no reports of liver injury. Therefore, administering a lower dose of tolvaptan over a shorter treatment regimen does not affect liver function in patients with preexisting liver disease, such as liver cirrhosis.
In summary, the results of this observational study show that tolvaptan is effective to treat refractory ascites and/or edema in decompensated cirrhotic patients and is, therefore, a promising aquaretic agent.
ACKNOWLEDGMENTS
The authors would like to acknowledge Health Interactions Asia Pacific Pte Ltd for language polishing and the writing assistance of the Korea Otsuka Pharmaceutical Co., Ltd. We also thank Ms. Park YJ for her support in acting as a liaison for medical writing assistance.
COMMENTS
Background
Tolvaptan, a new oral, selective vasopressin V2-receptor antagonist, is reported as an effective treatment for hyponatremia. Tolvaptan has a significant role in promoting aquaresis in patients with decompensated cirrhosis. However, the efficacy and safety of this drug for treating refractory ascites in cirrhotic patients and the effect(s) of tolvaptan use in patients with hepatorenal syndrome and/or hepatocellular carcinoma have not been studied previously.
Research frontiers
Numerous studies have reported the efficacy and safety of tolvaptan for the treatment of ascites and edema, and have demonstrated the efficacy of tolvaptan for treating hyponatremia in patients with decompensated cirrhosis.
Innovations and breakthroughs
This study shows that the combination of tolvaptan with diuretics is more effective than standard treatment approaches for refractory ascites in decompensated cirrhotic patients with or without further complications, such an hepatocellular carcinoma and/or hepatorenal syndrome.
Applications
Tolvaptan is a promising aquaretic agent that can be used to treat refractory ascites and/or edema in decompensated cirrhotic patients.
Terminology
Refractory ascites are defined as 1 wk of unresponsiveness to restrictions on salt intake and high-dose diuretics (more than 160 mg/d of furosemide and 200 mg/d of spironolactone) or recurrence occurring within 2 wk post-therapeutic paracentesis (removal of 3000-5000 mL of ascites fluid per treatment) and intermittent albumin infusion (10-20 g/d).
Peer review
This manuscript is the first reported open study showing that a short term (4-15 d) therapy with 15 mg/d of tolvaptan for refractory ascites in decompensated cirrhotic patients, as well as in patients with hepatorenal syndrome type 2 and hepatocellular carcinoma, is effective and safe. This is an interesting study.
Footnotes
Supported by The Program of Beijing Science and Technology Commission, No. D131100005313004; and the Beijing High-Level Talent Academic Leader/Personnel Training Programs awarded to Ding HG, 2011-2-19; Li B, 2013-3-072; and Li L, 2013-3-073
P- Reviewer: Guglielmi FW, Satapathy SK, Trapero-Marugan M, Wong GLH S- Editor: Gou SX L- Editor: Stewart G E- Editor: Wang CH
References
- 1.Gordon FD. Ascites. Clin Liver Dis. 2012;16:285–299. doi: 10.1016/j.cld.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651–1653. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 3.Bhogal H, Sanyal AJ. Treatment of refractory ascites. Clin Liver Dis. 2013;2:140–142. doi: 10.1002/cld.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singhal S, Baikati KK, Jabbour II, Anand S. Management of refractory ascites. Am J Ther. 2012;19:121–132. doi: 10.1097/MJT.0b013e3181ff7a8b. [DOI] [PubMed] [Google Scholar]
- 5.Moore CM, Van Thiel DH. Cirrhotic ascites review: Pathophysiology, diagnosis and management. World J Hepatol. 2013;5:251–263. doi: 10.4254/wjh.v5.i5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu C, Sharma N, Saab S. Hyponatremia: clinical associations, prognosis, and treatment in cirrhosis. Exp Clin Transplant. 2013;11:3–11. doi: 10.6002/ect.2012.0147. [DOI] [PubMed] [Google Scholar]
- 7.Josiassen RC, Curtis J, Filmyer DM, Audino B, Skuban N, Shaughnessy RA. Tolvaptan: a new tool for the effective treatment of hyponatremia in psychotic disorders. Expert Opin Pharmacother. 2010;11:637–648. doi: 10.1517/14656561003610656. [DOI] [PubMed] [Google Scholar]
- 8.Gaglio P, Marfo K, Chiodo J. Hyponatremia in cirrhosis and end-stage liver disease: treatment with the vasopressin V₂-receptor antagonist tolvaptan. Dig Dis Sci. 2012;57:2774–2785. doi: 10.1007/s10620-012-2276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaida I, Yamashita S, Kobayashi T, Komatsu M, Sakai T, Komorizono Y, Okada M, Okita K. Efficacy and safety of a 14-day administration of tolvaptan in the treatment of patients with ascites in hepatic oedema. J Int Med Res. 2013;41:835–847. doi: 10.1177/0300060513480089. [DOI] [PubMed] [Google Scholar]
- 10.Okita K, Kawazoe S, Hasebe C, Kajimura K, Kaneko A, Okada M, Sakaida I. Dose-finding trial of tolvaptan in liver cirrhosis patients with hepatic edema: A randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:83–91. doi: 10.1111/hepr.12099. [DOI] [PubMed] [Google Scholar]
- 11.Sakaida I, Kawazoe S, Kajimura K, Saito T, Okuse C, Takaguchi K, Okada M, Okita K. Tolvaptan for improvement of hepatic edema: A phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:73–82. doi: 10.1111/hepr.12098. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blei AT, Córdoba J. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968–1976. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 14.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leiva JG, Salgado JM, Estradas J, Torre A, Uribe M. Pathophysiology of ascites and dilutional hyponatremia: contemporary use of aquaretic agents. Ann Hepatol. 2007;6:214–221. [PubMed] [Google Scholar]
- 16.Schrier RW. Decreased effective blood volume in edematous disorders: what does this mean? J Am Soc Nephrol. 2007;18:2028–2031. doi: 10.1681/ASN.2006111302. [DOI] [PubMed] [Google Scholar]
- 17.Hou W, Sanyal AJ. Ascites: diagnosis and management. Med Clin North Am. 2009;93:801–17, vii. doi: 10.1016/j.mcna.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Chen RP, Zhu Ge XJ, Huang ZM, Ye XH, Hu CY, Lu GR, Lu de Y, Phemba IL. Prophylactic use of transjugular intrahepatic portosystemic shunt aids in the treatment of refractory ascites: metaregression and trial sequential meta-analysis. J Clin Gastroenterol. 2014;48:290–299. doi: 10.1097/MCG.0b013e3182a115e9. [DOI] [PubMed] [Google Scholar]
- 19.Berl T, Quittnat-Pelletier F, Verbalis JG, Schrier RW, Bichet DG, Ouyang J, Czerwiec FS; SALTWATER Investigators. Oral tolvaptan is safe and effective in chronic hyponatremia. J Am Soc Nephrol. 2010;21:705–712. doi: 10.1681/ASN.2009080857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cárdenas A, Ginès P, Marotta P, Czerwiec F, Oyuang J, Guevara M, Afdhal NH. Tolvaptan, an oral vasopressin antagonist, in the treatment of hyponatremia in cirrhosis. J Hepatol. 2012;56:571–578. doi: 10.1016/j.jhep.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 21.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C; SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 22.Miyazaki T, Fujiki H, Yamamura Y. Tolvaptan, an orally active non-peptide arginine vasopressin V2 receptor antagonist, reduces ascites in rats with chronic liver injury. Hepatol Res. 2013;43:1224–1230. doi: 10.1111/hepr.12073. [DOI] [PubMed] [Google Scholar]
- 23.Habib S, Boyer TD. Vasopressin V2-receptor antagonists in patients with cirrhosis, ascites and hyponatremia. Therap Adv Gastroenterol. 2012;5:189–197. doi: 10.1177/1756283X12437357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okita K, Sakaida I, Okada M, Kaneko A, Chayama K, Kato M, Sata M, Yoshihara H, Ono N, Murawaki Y. A multicenter, open-label, dose-ranging study to exploratively evaluate the efficacy, safety, and dose-response of tolvaptan in patients with decompensated liver cirrhosis. J Gastroenterol. 2010;45:979–987. doi: 10.1007/s00535-010-0240-6. [DOI] [PubMed] [Google Scholar]
- 26.Sakaida I, Okita K. Correlation between changes in bodyweight and changes in ascites volume in liver cirrhosis patients with hepatic edema in short-term diuretic therapy. Hepatol Res. 2014;44:735–739. doi: 10.1111/hepr.12171. [DOI] [PubMed] [Google Scholar]


