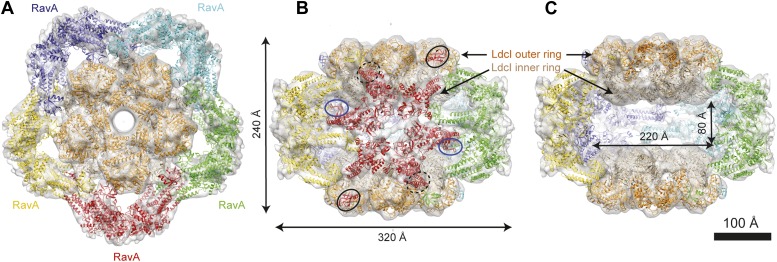
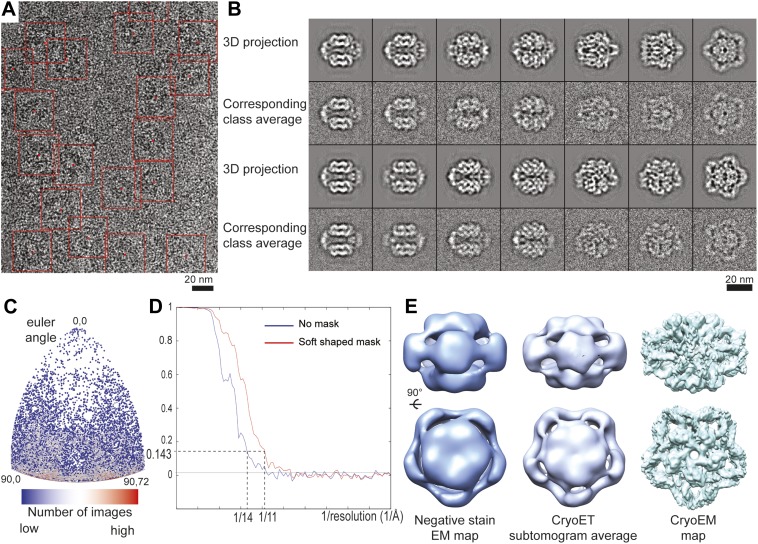
Figure 1. Cage-like architecture of the LdcI–RavA complex (11 Å resolution).
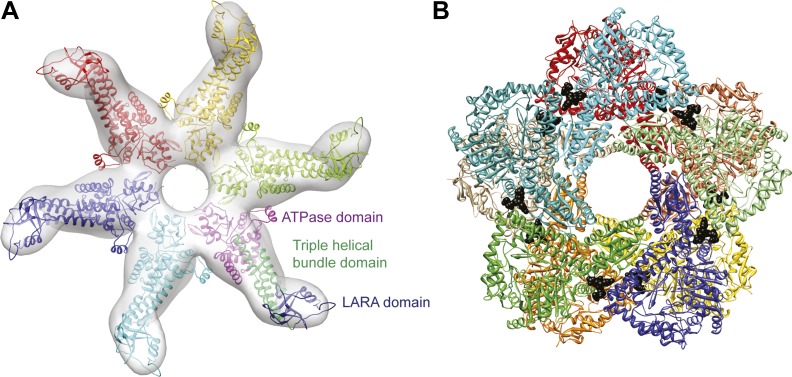
(A) Top view with LdcI facing the reader, (B) Side view with RavA facing the reader. For this RavA hexamer, LARA domain positions are indicated by ellipses (solid black for the two LARA domains interacting with the inner LdcI rings from above, dotted black for the two LARA domains interacting with the outer LdcI rings from underneath and invisible from this orientation, solid dark blue for the LARA domains interacting equatorially with the triple helical domains of adjacent RavA monomers). (C) Side cut-away view. Complex dimensions are indicated.