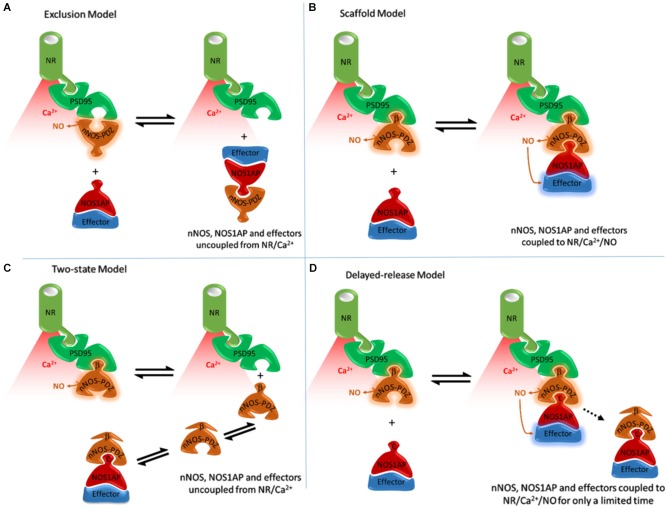
Figure 1.
Alternative models of NOS1AP interaction with nNOS and their anticipated consequences to exposure of NOS1AP effector to nitric oxide. (A) The Exclusion Model, based on Jaffrey et al. (1998) and Eastwood (2005). Binding of PSD95 to nNOS excludes binding of NOS1AP by PDZ-PDZ interaction and direct competition and vice versa. A PDZ-PDZ interaction was originally envisioned (Jaffrey et al., 1998), but this is consistent neither with structural nor functional data. Coupling of nNOS to NMDAR/Ca2+-influx is important for activation (Aarts et al., 2002; Ishii et al., 2006). Therefore in all schemes nNOS, when coupled to NMDAR (via PSD95) is shown producing NO (active), whereas nNOS displaced from NMDAR/Ca2+-influx (red shading) is depicted without NO production. In this model the nNOS/NOS1AP complexes with effectors such as DexRas would not be directly localized to the receptor and calcium influx-associated NO produced. (B) The Scaffold Model, based on Christopherson et al. (1999) and Li et al. (2013). Binding of nNOS β-finger to PSD95 facilitates an extended complex incorporating NOS1AP (or other ligands with C-terminal motifs). This model places nNOS close to the source of calcium influx, and NOS1AP effectors close to NO produced. This is consistent with NOS1AP mediating actions of NMDAR activated nNOS (Fang et al., 2000; Cheah et al., 2006; Li et al., 2013). But it is not consistent with cell-free experiments in which NOS1AP competes with PSD95 for binding nNOS (Jaffrey et al., 1998). (C) The Two-state model. The extended PDZ domain of nNOS is proposed to exist in two conformational states. One can bind PSD95 not NOS1AP, the other NOS1AP not PSD95. This could explain competition between PSD95 and NOS1AP. This model, however, places the nNOS-NOS1AP complex at a distance from the NMDA receptor, limiting activation of nNOS in the nNOS-NOS1AP complex. This is not consistent with NOS1AP mediating NMDAR/nNOS-dependent pathways (Fang et al., 2000; Cheah et al., 2006; Li et al., 2013). (D) The Delayed-release model. Here NOS1AP can interact with the unoccupied PDZ pocket seen in Figure 2, allowing the coupling of NMDAR/nNOS signaling to NOS1AP dependent pathways. But undefined mechanisms gradually lead to the loss of PSD95 binding by the beta-finger, presumably via conformational changes, resulting in delayed dissociation of the nNOS-NOS1AP complex from the receptor. In this model, the nNOS/NOS1AP effector complex is localized with the receptor and associated calcium influx for a limited time only. This model potentially explains the apparently conflicting data on NOS1AP function.