Abstract
This study was designed to evaluate the neuroprotective effects of Morinda citrifolia L. (Rubiaceae), commonly known as noni, and memantine (a N-methy-D-aspartate receptor inhibitor) on hydrocephalus-induced neurodegenerative disorders. Kaolin was injected into the cistern magna of male adult New Zealand rabbits to establish a hydrocephalus animal model. Memantine (20 mg/kg, intraperitoneally; memantine-treated group) or noni (5 mL/kg, intragastrically; noni-treated group) was administered daily for 2 weeks. Microtubule-associated protein-2 and caspase-3 immunohistochemistry were performed to detect neuronal degeneration and apoptosis in the periventricular tissue of the fourth ventricle of rabbits. Microtubule-associated protein-2 staining density was significantly decreased in the hydrocephalic group, while the staining density was significantly increased in the memantine- and noni-treated groups, especially in the noni-treated group. Noni treatment decreased the number of caspase-3-positive cells in rabbits with hydrocephalus, while memantine had no effect. These findings suggest that noni exhibits more obvious inhibitory effects on hydrocephalus-induced neurodegenerative disorders than memantine in periventricular tissue of the fourth ventricle.
Keywords: neural regeneration, neurodegenerative disease, traditional Chinese medicine, hydrocephalus, Morinda citrifolia L. (noni), memantine, fourth ventricle, periventricular tissue, microtubule-associated protein-2, caspase-3, apoptosis, grants-supported paper, photographs-containing paper, neuroregeneration
Research Highlights
-
(1)
Morinda citrifolia L. (Rubiaceae), known as noni, has been extensively used in folk medicine in Polynesia and tropical parts of eastern Asia and Australia, and contains some antioxidative or anti-inflammatory ingredients.
-
(2)
This study monitored the microtubule-associated protein-2, a major component of the cytoskeleton, and the upregulation of caspase-3 as detection indices to validate that noni and memantine protect against hydrocephalus-induced neurodegenerative disorders.
-
(3)
Memantine, a N-methyl-D-aspartate receptor inhibitor, has been used for the treatment of Alzheimer's disease. Results from this study demonstrate that memantine can alleviate hydrocephalus-induced neurodegenerative disorders.
-
(4)
Noni exhibits more obvious inhibitory effects on hydrocephalus-induced neurodegenerative disorder than memantine in periventricular tissue of the fourth ventricle.
INTRODUCTION
Hydrocephalus is a condition characterized by impaired secretion, circulation and resorption of cerebrospinal fluid, resulting in ventricular dilatation[1]. This distortion has deleterious effects that include gliosis, inflammatory responses[2], neurodegeneration[3], axonal damage[1], demyelination[4], impaired cerebral blood flow[5], and altered clearance of proteins[6] and toxins[7]. However, the mechanisms underlying these deficits are not fully understood[2,8]. Ventriculoperitoneal shunt placement is a worldwide accepted procedure for the treatment of hydrocephalus. Nevertheless, the shunt procedure has various complications. Common presentations of this complication are development of an abdominal mass, abdominal pain, and intestinal obstruction[9,10,11]. In the several cases, shunt placement into the fourth ventricle is a widely used approach despite the well-known risks such as shunt dysfunction and dislocation. However, shunt placement does not reestablish the physiological drainage of the fourth ventricle[12,13,14]. To the best of our knowledge, there are currently no drugs for the treatment of hydrocephalus. Further drug studies may prevent unwanted complications.
Programmed cell death, or apoptosis, occurs in response to many different environmental stimuli[15,16]. Caspase activation is the “point of no return” during programmed cell death[17]. The presence of the activated form of caspase-3 marks the point of no return within the complex apoptotic signaling cascade[18]. Caspase-3 has been implicated in neurodegenerative processes[19]. Caspase-3 is a key protein involved in the classical apoptosis mechanism in neurons, as in many other cellular types[20]. Studies of experimental models suggested that activated caspase-3 is a reliable indicator of apoptotic rate[21,22]. The programmed cell death mechanisms in hydrocephalus are generally unclear. There is also a limited number of animal studies in this field, but some evidence points towards apoptotic mechanisms contributing to hydrocephalus[8,23]. Caspase-3 activation has not been sufficiently investigated in hydrocephalic brains[24].
Microtubule-associated protein-2 plays a significant role in neurite outgrowth[25,26] and plasticity of the nervous system[27,28,29]. As downregulation of microtubule-associated protein-2 significantly increases vulnerability to insults, preservation of microtubule-associated protein-2 protein by antioxidants are associated with neuronal survival[30,31,32]. Either central nervous system trauma or neurodegeneration leads to cytoskeletal alterations affecting microtubules and neurofilaments, in particular, the loss of microtubule-associated protein-2, a major component of the cytoskeleton[33,34,35,36,37].
Morinda citrifolia L. (Rubiaceae, Noni), has been extensively used in folk medicine in Polynesia and tropical parts of eastern Asia and Australia[38]. Noni juice has various pharmacological properties, including antioxidant[39,40,41,42,43] and anti-inflammatory effects[44,45]. Thus, it is proposed that the antioxidative and anti-inflammatory properties of noni juice may provide a protective effect against the neurodegeneration caused by hydrocephalus.
Memantine is a N-methyl-D-aspartate receptor inhibitor that is neuroprotective and is approved for human use in the inhibition of neurodegeneration in Alzheimer's disease[46,47]. It is also currently under evaluation in clinical and experimental trials, for use as a treatment in a number of other neurodegenerative diseases[48,49]. This is the reason why memantine may prevent against neurodegenerative disorders caused by hydrocephalus. The aim of this study was to investigate the neuroprotective properties of noni and memantine using microtubule-associated protein-2 and caspase-3 immunoreactivity in the periventricular tissue of the fourth ventricle in hydrocephalic rabbits.
RESULTS
Quantitative analysis of animals
Twenty-four adult male New Zealand white rabbits were initially included in the study and divided into four equal groups: control, hydrocephalic, memantine-treated hydrocephalic and noni-treated hydrocephalic groups. Hydrocephalus was induced by kaolin injection into the cisterna magna of all animals with the exception of the control group, and memantine was given intraperitoneally at a daily dose of 20 mg/kg body weight and noni was given intragastrically 5 mL/kg for 2 weeks. All 24 rabbits were included in the final analysis.
MRI displayed ventricular dilatation after hydrocephalus induction
In MRI examinations, T2-weighted images of the brain in the coronal plane were obtained. MRI displayed ventricular dilatation 2 weeks after hydrocephalus induction (Figure 1A). MRI of the control group showed normal ventricular structure (Figure 1B).
Figure 1.
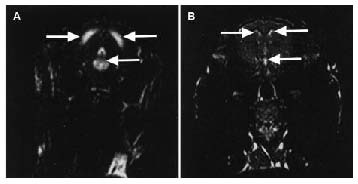
MRI of ventricular dilatation after hydrocephalus induction in rabbits.
(A) An example of MRI displaying ventricular dilatation 2 weeks after hydrocephalus induction. Severe ventricular enlargement is apparent. (B) A typical MRI of a control rabbit revealing normal ventricular structure. The ventricles are indicated by arrows.
Microtubule-associated protein-2 immunoreactivity in periventricular tissue of the fourth ventricle after hydrocephalus induction in rabbits
The changes in microtubule-associated protein-2 staining density in the periventricular region of hydrocephalus rats were evaluated. Semi-quantitative analysis of staining density of neurons in the periventricular tissue showed a significant reduction in immunoreactivity in the hydrocephalic group as compared with the control group (P < 0.001; Figures 2A, E). However, memantine and noni administration significantly enhanced microtubule-associated protein-2-immunopositive staining (Figures 2B, C) in the hydrocephalic group (P < 0.001). In the hydrocephalic group, administration of kaolin resulted in a loss of microtubule-associated protein-2 immunoreactivity that was particularly obvious in the periventricular tissue of the fourth ventricle (Figure 2D). The reduction in microtubule-associated protein-2 immunoreactivity was largely eliminated in hydrocephalic rabbits after noni administration (Figure 2E).
Figure 2.

Microtubule-associated protein-2 (MAP-2) immunoreactivity in the periventricular tissue of the fourth ventricle of rabbits after noni and memantine treatments.
(A) In the control group, MAP-2 labeled neurons are indicated by arrowheads. The memantine-treated group (B) and the noni-treated group (C) showing MAP-2 staining in the periventricular tissue of the fourth ventricle (IV). (D) In the hydrocephalic group, there was a decrease in MAP-2 staining. Asterisks point to holes adjacent to the ventricular surface in the periventricular tissue. Scale bar: 100 μm. (E) Graph showing the percentage of MAP-2 antibody-stained area in relation to the whole area. n = 6 rabbits per group, values are expressed as the mean ± SEM, and were compared by one-way analysis of variance with the post hoc Tukey test. Noni-treated group vs. control group, memantine group, and hydrocephalic group, aP < 0.001; memantine group vs. control group and hydrocephalic group, bP < 0.001; hydrocephalic vs. control group, cP < 0.001.
Cell apoptosis in periventricular tissue of the fourth ventricle after hydrocephalus induction in rabbits
Caspase-3 labeling showed negative or very few positive cell staining in the control group (Figure 3A). There was a significant decrease in the number of caspase-3 labeled cells in the noni group when compared with the hydrocephalic group (Figures 3C, E; P < 0.001). In addition, the number of caspase-3 labeled cells in the noni group was significantly higher than that in the memantine group (P < 0.001). However, the number of caspase-3 labeled cells was greatest in the memantine group (Figure 3B; P < 0.001). Hydrocephalus was characterized by severe ependymal damage, the development of holes directly adjacent to the ventricular surface in the periventricular tissue, and the formation of periependymal edema (Figure 3D).
Figure 3.
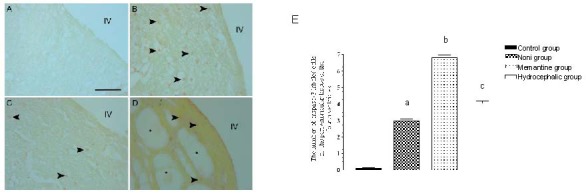
Caspase-3 immunoreactivity in the periventricular tissue of the fourth ventricle of rabbits receiving noni and memantine treatment.
(A) There was no anti-caspase-3 immunoreactivity in the control group. (B) There was a large number of caspase-3 labeled cells (arrowheads) in the memantine group. (C) There was a significant decrease in the number of caspase-3 labeled cells (arrowheads) in the noni group when compared with the hydrocephalic group. (D) In the hydrocephalic group, the decrease in the number of caspase-3 labeled cells (arrowheads) was probably due to rapid loss of apoptotic cells. Asterisks indicate developed holes in the periventricular tissue. IV: The fourth ventricle. Scale bar: 100 μm.
(E) Graph showing the number of caspase-3-labeled cells in the periventricular tissue of the fourth ventricle. n = 6 rabbits per group and values are expressed as mean ± SEM. The one-way analysis of variance with the post hoc Tukey test was used. Noni group vs. control group, memantine group, and hydrocephalic group, aP < 0.001; memantine group vs. control group and hydrocephalic group, bP < 0.001; hydrocephalic vs. control group, cP < 0.001.
DISCUSSION
Hydrocephalus is a common disorder of defective cerebrospinal fluid turnover[50]. It is generally caused from a disturbance of production, flow, or absorption of cerebrospinal fluid, leading to an excessive accumulation of cerebrospinal fluid in the intracranial cavity of the brain[8,51].
Shunt surgery is a worldwide accepted procedure for treatment of hydrocephalus[11,51,52]. However, shunt failure and complications are common and may require multiple surgical procedures during a patient's lifetime. Infection, obstruction, and overdrainage are the major causes of shunt malfunction resulting in shunt revision in hydrocephalic patients. Other causes include, but are not limited to, proximal shunt failure, distal shunt failure, shunt dysfunction, and valve problems. Many of these complications are believed to be directly related to surgical procedure and patient management[52]. Thus, the management of hydrocephalus with multiple shunt failures is still a challenging problem in neurosurgery. Nevertheless, it appears that shunt surgery remains the best treatment option for hydrocephalic patients. Alternatively, improved outcomes may be achieved in these patients with the use of antibiotic impregnated shunts, which could help lower the infection rate for patients and minimize shunt complications. However, the high proportion of patients experiencing shunt failure after shunt placement is still a concern. For this reason, we aimed to determine whether noni and memantine may be effective as a potential treatment strategy for hydrocephalus.
Neuronal microtubules are known to be rapidly downregulated by almost all forms of trauma and neurodegenerative diseases, such as stress, injury, Alzheimer's disease, Parkinson's disease and Lewy body disease[34,37]. Central nervous system traumatic and neurodegenerative disorders lead to cytoskeletal alterations affecting microtubules and neurofilaments, in particular the loss of microtubule-associated protein-2[33,35]. The signs of neurodegenerative disorders are associated with marked loss or reduction in immunoreactivity for microtubule-associated protein-2. Loss of microtubule-associated protein-2 could lead to cytoskeletal changes and neuronal death in Alzheimer's disease[34]. A loss of microtubule-associated protein-2 immunoreactivity also occurs in a variety of pathological conditions, such as exposure to excess calcium influx and calpain activation, N-methyl-D-aspartate-activation, oxidative stress, and dephosphorylation[30,34].
Several studies found that the monoclonal antibody against microtubule-associated protein-2 may recognize a determinant present in neurofibrillary tangles[53,54]. The microtubule-associated protein-2 monoclonal antibody could clearly mark neurofibrillary tangles in the most infected region of brain tissue of patients with Alzheimer's disease[55,56], and the polyclonal antibody may label abnormal neurites around senile plaques[57,58], suggesting that microtubule-associated protein-2 may be involved in the occurrence of neurodegenerative diseases caused by the neurotoxic effects of amyloid-β[59]. During the progression of Alzheimer's disease, there is a mechanism which might interfere with the role of stable microtubules of microtubule-associated protein-2, leading to cytoskeletal changes and neuronal death, and the existence of certain factors that influence microtubule-associated protein-2 regulation of organelle transport[34]. We also observed decreased microtubule-associated protein-2 immunoreactivity in the hydrocephalic group. These data suggest that loss of microtubule-associated protein-2 immunoreactivity indicates neurodegenerative disorders in the hydrocephalic group. Microtubule-associated protein-2 immunoreactivity in the noni and memantine groups seemed to be increased when compared with the hydrocephalic group. These data also suggest that noni and memantine may prevent neurodegenerative disorders in hydrocephalus.
Apoptosis plays a key role in central nervous system development, while in the adult brain it is involved in the pathogenesis of a number of diseases including neurodegenerative diseases and acute injury such as stroke[60]. Neuronal apoptosis plays an important role in Alzheimer's disease pathogenesis, and caspases seem to be involved in some upstream pathological events[61]. A preponderant role of the aberrant activation of intrinsic and extrinsic apoptotic pathways in Parkinson's disease pathogenesis has been suggested. The involvement of caspases 1 and 3 in apoptotic cell death has been proven using Parkinson's disease animal models[60]. Caspases cause neurodegenerative disorders in a large number of different diseases. In addition, caspases are proteases that irreversibly commit a cell on the pathway of death. Once activated, they cleave a range of critical cellular proteins, setting the point of no return. Thus, the presence of active caspase-3 is a good indicator of apoptosis[17]. Animal studies in hydrocephalus have shown that damage of neurons, axons, and oligodendrocytes is associated with apoptotic cell death[1,8,62]. Deren et al[8] observed changes in several genes involved in the apoptotic pathway whose interplay with other genes may result in an inflammatory response. Microarray analysis identified significant changes in the apoptosis pathway (10/69) genes.
Castejón[62] reviewed the ultrastructural pathology of the cerebral cortex in human hydrocephalus and compared this with experimental hydrocephalus. Myelination delay and axonal and oligodendroglial cell damage were reported in both human and experimental hydrocephalus. The nerve cell death in congenital hydrocephalus is related to the severity of brain edema, anoxic-ischemic conditions of brain parenchyma, oxidative stress, glutamate excitotoxicity, calcium overloads, and caspase dependent and independent mechanisms[62]. Felderhoff-Mueser et al[63] determined the levels of soluble Fas, soluble FasL, and activated caspase-3 in the cerebrospinal fluid of preterm infants with posthemorrhagic hydrocephalus. They found that soluble Fas was higher in patients with posthemorrhagic hydrocephalus and non-hemorrhagic hydrocephalus than controls. Nevertheless, the pro-apoptotic factors soluble FasL and activated caspase-3 did not differ between infants with hydrocephalus and control infants. However, the authors concluded that apoptosis does occur in the brains of infants with hydrocephalus. They hypothesized that soluble FasL and caspase-3 were absent because hydrocephalus is a more chronic process, and also the levels of these proteins may be too low in the cerebrospinal fluid of infants with posthemorrhagic hydrocephalus to be detected by their methods[63]. Therefore, caspase-3 immunohistochemistry was examined in the periventricular tissue of the fourth ventricle of noni- and memantine-treated hydrocephalic rabbits. We did not monitor apoptosis in specific cell types, but evaluated apoptosis in all cells in the periventricular tissue of the fourth ventricle of rabbits. We also examined neurodegeneration using microtubule-associated protein-2 immunoreactivity.
Recently, memantine prevented the deleterious effect of amyloid-beta 1–42 on synaptic plasticity and learning behavior in rats[64]. A large number of studies using in vitro and in vivo animal models demonstrated that memantine protects cerebrocortical neurons, cerebellar neurons, and retinal neurons from N-methyl-D-aspartate receptor-mediated excitotoxic damage. Memantine has acute and longer term neuroprotective effects on markers of synapse development in the immature rat brain, and studies investigating its effect on other neurodegenerative disorders, including human immunodeficiency virus-associated dementia, Huntington's disease, amyotrophic lateral sclerosis, and depression[48] are currently underway. We also found increased microtubule-associated protein-2 immunoreactivity in the memantine group as compared with the hydrocephalic group, whereas there was a significant increase in the number of caspase-3 labeled cells in the memantine group as compared with the hydrocephalic group. The decrease in the number of caspase-3 labeled cells was probably due to rapid loss of apoptotic cells in the hydrocephalic group. Therefore, enlarged extracellular space and edematous changes of the subependymal white matter were observed. Furthermore, several researchers suggested different mechanisms of neural cell death in hydrocephalus[24,62]. Apoptosis is partially controlled by the Fas/FasL system and a protease known as caspase-3[24]. Neural cell death can also occur in relation with the severity of brain edema, anoxic-ischemic conditions of the brain parenchyma, oxidative stress, activation of N-methyl-D-aspartate receptors, calcium overload, and caspase dependent and independent mechanisms[62]. This neuroprotective mechanism is most likely mediated by different neuroprotective pathways of memantine. Memantine is a partial noncompetitive antagonist of N-methyl-D-aspartate receptors. The blockade of N-methyl-D-aspartate receptor-mediated excitotoxicity can help preserve neuronal structure and function. N-methyl-D-aspartate antagonists cause adverse side effects ranging from memory dysfunction and psychotic reactions in humans to acute injury and/or death of neurons in the animal brain[65]. Treatment with low doses of memantine protects against N-methyl-D-aspartate-induced toxicity in vitro[66], as well as neurotoxicity and learning deficits in chemical lesion models and neurodegenerative disease models[67,68,69]. However, high doses of memantine produced deleterious effects. Chronic treatment with a high dose (30 mg/kg) of memantine in vivo increased striatal neuronal degeneration in a Huntington's disease mouse model; thus, further investigations on the dose effect of memantine, especially in different neuronal types and brain regions, is warranted[70]. N-methyl-D-aspartate receptors are most affected in Alzheimer's disease in the parietal and temporal cortex and other neuropathological changes associated with this disease are prominent in these regions as well.
The increased metabolism seen in the inferior parietal and temporal cortex with memantine treatment may be due to the N-methyl-D-aspartate receptor-mediated benefits in these specific regions, such as increased local synaptic activity[71]. In conclusion, memantine may prevent the development of neurodegenerative disorders and apoptosis induced by hydrocephaly if used in appropriate low doses, but the pharmacological and molecular mechanisms should be fully explored.
Noni contains some antioxidative or anti-inflammatory ingredients[44]. The anti-inflammatory effect of noni juice can be explained by the presence of flavonoid and coumarin molecules[72]. In fact, the flavonoid and coumarin molecules induce their inhibitory effects on edema through the nitric oxide and prostaglandins E2 pathways[38]. The anti-oxidant properties of noni juice are probably associated with the phenolic compounds iridoids and ascorbic acid[38,73]. The noni juice showed hypolipidemic and antioxidative effects on high-fat/cholesterol-dietary hamsters. It is proposed that the antioxidative[43] and anti-inflammatory properties[44,45] of noni may provide a protective effect against neuronal damage caused by hydrocephalus. Image analysis showed that the staining density of microtubule-associated protein-2 following noni administration was greater than in hydrocephalic rabbits, indicating that noni reduced loss of microtubule- associated protein-2 immunostaining density. The number of caspase-3- labeled cells was lower in the noni group than in the memantine and hydrocephalic groups. Thus, the protective effect of noni was demonstrated using both caspase-3 and microtubule- associated protein-2 immunostaining on hydrocephalic model rabbits.
We quantified microtubule-associated protein-2 immunostaining density as a marker of changes to the neuronal cytoskeleton. Our results showed that memantine and noni inhibited apoptosis, and precluded the loss of microtubule-associated protein-2. Neurodegeneration following hydrocephalus was accompanied by changes in microtubule-associated protein-2 immunoreactivity, which was characterized as a decrease in microtubule-associated protein-2 immunostaining. Antigenic sites for microtubule-associated protein-2 are present in the dendritic tree[74] and neuronal cell bodies[30]. In the hydrocephalic rabbits, microtubule-associated protein-2 immunoreactivity dramatically decreased in the periventricular tissue. This finding suggests pronounced dendrite degeneration following hydrocephalus. In contrast, the memantine and noni groups showed significantly increased staining with microtubule-associated protein-2 compared with the hydrocephalic group. Hydrocephalic rabbits showed reduced immunostaining for microtubule-associated protein-2 in neurons, corresponding to cytoskeletal alteration and neurodegeneration, whereas memantine and noni treatment prevented the loss of microtubule-associated protein-2.
In conclusion, our results demonstrated the neuroprotective effects of noni and memantine upon periventricular tissue of the fourth ventricle in hydrocephalic rabbits, but further studies are needed to establish noni and memantine as candidate neuroprotective drugs for humans.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
Experiments were performed from November 2009 to September 2011 at the Laboratory of Histology and Embryology, Faculty of Medicine, Kocaeli University, Turkey.
Materials
Twenty-four male New Zealand white rabbits, weighing 3 ± 0.19 kg, aged 4–5 months, were provided by the Experimental Animal Center of Kocaeli University, Turkey. All experimental protocols were performed according to the Guidance Suggestions for the Care and Use of Laboratory Animals issued by the Ministry of Science and Technology of Turkey.
Noni was provided by Morinda International Inc., Thailand Branch. Product name: Tahitian Noni Original Concentrate. Ingredients: 100% concentrated noni fruit, total 30 mL, 1 fluid ounce.
Methods
Hydrocephalus induction
Animals were anesthetized by intramuscular injection of a mixture of ketamine (50 mg/kg) and xylazine (5 mg/kg). The neck of each animal was shaved and flexed, and a median occipitocervical junction incision was made. The occipital and cervical paravertebral muscles were dissected and the atlanto-occipital membrane was identified. An insulin injector of 23 G was introduced into the cisterna magna, 0.5 mL cerebrospinal fluid was removed under aseptic conditions, and 0.5 mL (500 mg/mL in 0.09 % (w/v) NaCl) kaolin suspension (kaolin hydrated aluminum silicate, K-7375, Sigma, St. Louis, MO, USA) was slowly injected into the cisterna magna in rabbits. The atlanto-occipital membrane was identified for sham-operated controls in the control group, and 0.5 mL cerebrospinal fluid was removed and then injected back into the cisterna magna. Animals were allowed to survive for 2 weeks.
Animals in the memantine group were intraperitoneally administered 20 mg/kg memantine (Ebixa®, Lundbeck Inc., Istanbul, Turkey) in distilled water, once per day, for 2 weeks. Animals in the noni group were intragastrically 5 mL/kg noni, once per day, for 2 weeks.
MRI examinations
Cranial MRI were acquired from all animals using a Philips Intera 1.5 T MR (Philips, Best, the Netherlands) after 2 weeks to measure dilation in the ventricular system. The coronal slices from the fourth ventricle were obtained to verify ventricular dilation and development of hydrocephalus. MRI results revealed that one rabbit from the memantine group and one from the noni group failed to develop ventricular dilatation, and these animals were excluded from the study. Only rabbits with significant ventriculomegaly (severe ventricular enlargement) were used in this study.
Brain tissue preparation
After 2 weeks, all experimental rabbits were perfused first with PBS under ethyl ether anesthesia, and then with buffered 4% (w/v) paraformaldehyde. Brains were dissected and postfixed in the same fixative. Following fixation, coronal blocks of the cerebellar cortex were embedded in paraffin and sectioned at 5 μm. The paraffin sections were used for microtubule associated protein-2 and caspase-3 immunostaining as described below. Serial cross-sections through the caudal part of the fourth ventricle and cerebellum were defined according to the Stereotaxic Atlas of the New Zealand Rabbit's Brain[75]. Periventricular tissue of the fourth ventricle was taken within ~200 μm of ventricular wall[76].
Immunohistochemical staining
Paraffin sections were immunostained using the avidin-biotin peroxidase (mouse ABC staining system, sc-2017, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and horseradish peroxidase-streptavidin method (for mouse and rabbit primary antibodies, KP-50DR, Diagnostic Bio Systems, Pleasanton, CA, USA) for the anti-microtubule-associated protein-2 mouse monoclonal (1:500 dilution; ab28032, Abcam, Cambridge, UK) and anti-caspase-3 rabbit polyclonal antibodies (1:50 dilution; CPP32, Diagnostic Bio Systems). The paraffin- embedded tissue slices were deparaffinized with xylene. The endogenous peroxidase activity was inhibited by incubation in 0.3% (v/v) hydrogen peroxide in methanol. The tissue slices were hydrated with graded alcohol, treated with 10% (v/v) normal serum, and then incubated with the primary antibodies at 4°C overnight. They were then incubated with biotinylated anti-mouse IgG or the biotinylated anti-rabbit IgG for 30 minutes at room temperature, then with avidin biotinylated horseradish peroxidase or streptavidin horseradish peroxidase in 10% (v/v) normal goat serum for 30 minutes at room temperature. The slices were then visualized using 3-amino-4- ethylcarbazole as the chromogen. Negative controls consisted of tissue sections incubated without primary antibody. Finally, the sections were mounted for quantitative analysis.
Evaluation of microtubule-associated protein-2 staining
Images of the immunohistochemically stained sections for microtubule-associated protein-2 were captured with a Leica DFC290 HD color digital camera mounted on a Leica DM1000 microscope (Leica, Nussloch, Germany) using a 20 × objective and stored as Tagged Image File Format. Images were then analyzed with Image J software. In each image, the parameters measured by the image analysis program were the percentage of antibody-stained area in relation to the whole area.
Counting of caspase-3 labeled cells
The presence of cells undergoing apoptosis was determined by immunohistochemical detection of caspase-3. We randomly selected four 200 × 200 μm2 fields in the three coronal sections of the periventricular tissue of the fourth ventricle for each rabbit. Caspase-3 labeled cells were counted.
Statistical analysis
Statistical analysis was performed using the computer software program SPSS for Windows (SPSS, Chicago, IL, USA). Neuron counts and caspase-3 immunoreactivity values of the groups were analyzed by one-way analysis of variance with the post hoc Tukey test for intergroup comparisons. All P values less than 0.05 were considered to be statistically significant. The data were expressed as mean ± SEM.
Footnotes
Funding: This study was sponsored by a grant from the Education and Research Foundation of Faculty of Medicine, Kocaeli University, No. 2009/45.
Conflicts of interest: None declared.
Ethical approval: All experimental protocols received full approval from the Animal Ethical Committee of Kocaeli University, Turkey, No.15/2-2009.
(Edited by Li Y, Min Y/Song LP)
REFERENCES
- [1].Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Dev Disabil Res Rev. 2010;16(1):16–22. doi: 10.1002/ddrr.94. [DOI] [PubMed] [Google Scholar]
- [2].Xu H, Zhang SL, Tan GW, et al. Reactive gliosis and neuroinflammation in rats with communicating hydrocephalus. Neuroscience. 2012;30(218):317–325. doi: 10.1016/j.neuroscience.2012.05.004. [DOI] [PubMed] [Google Scholar]
- [3].Tarnaris A, Toma AK, Pullen E, et al. Cognitive, biochemical, and imaging profile of patients suffering from idiopathic normal pressure hydrocephalus. Alzheimers Dement. 2011;7(5):501–508. doi: 10.1016/j.jalz.2011.01.003. [DOI] [PubMed] [Google Scholar]
- [4].Cardoso EJ, Lachat JJ, Lopes LS, et al. Changes caused by hydrocephalus, induced by kaolin, in the corpus callosum of adult dogs. Acta Cir Bras. 2011;26(2):8–14. doi: 10.1590/s0102-86502011000800003. [DOI] [PubMed] [Google Scholar]
- [5].Kolarovszki B, Zubor P, Kolarovszka H, et al. The assessment of intracranial dynamics by transcranial Doppler sonography in perioperative period in paediatric hydrocephalus. Arch Gynecol Obstet. 2013;287(2):229–238. doi: 10.1007/s00404-012-2576-z. [DOI] [PubMed] [Google Scholar]
- [6].Silverberg GD, Miller MC, Machan JT, et al. Amyloid and Tau accumulate in the brains of aged hydrocephalic rats. Brain Res. 2010;4(1317):286–296. doi: 10.1016/j.brainres.2009.12.065. [DOI] [PubMed] [Google Scholar]
- [7].Owler BK, Pitham T, Wang D. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res. 2010;7:15. doi: 10.1186/1743-8454-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deren KE, Packer M, Forsyth J, et al. Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Exp Neurol. 2010;226(1):110–119. doi: 10.1016/j.expneurol.2010.08.010. [DOI] [PubMed] [Google Scholar]
- [9].Buyukyavuz BI, Duman L, Karaaslan T, et al. Hyponatremic seizure due to huge abdominal cerebrospinal fluid pseudocsyt in a child with ventriculoperitoneal shunt: a case report. Turk Neurosurg. 2012;22(5):656–658. doi: 10.5137/1019-5149.JTN.3978-10.1. [DOI] [PubMed] [Google Scholar]
- [10].Vybíhal V, Svoboda T, Procházka V, et al. Comparison of laparotomic and laparoscopic techniques for implantation of the peritoneal part of the shunt in the treatment of hydrocephalus. Rozhl Chir. 2012;91(6):305–310. [PubMed] [Google Scholar]
- [11].Reddy GK. Ventriculoperitoneal shunt surgery and the incidence of shunt revision in adult patients with hemorrhage-related hydrocephalus. Clin Neurol Neurosurg. 2012;114(9):1211–1216. doi: 10.1016/j.clineuro.2012.02.050. [DOI] [PubMed] [Google Scholar]
- [12].Lee M, Leahu D, Weiner HL, et al. Complications of fourth-ventricular shunts. Pediatr Neurosurg. 1995;22:309–313. doi: 10.1159/000120921. [DOI] [PubMed] [Google Scholar]
- [13].Pang D, Zwienenberg-Lee M, Smith M, et al. Progressive cranial nerve palsy following shunt placement in an isolated fourth ventricle: case report. J Neurosurg. 2005;102:326–331. doi: 10.3171/ped.2005.102.3.0326. [DOI] [PubMed] [Google Scholar]
- [14].Armbruster L, Kunz M, Ertl-Wagner B, et al. Microsurgical outlet restoration in isolated fourth ventricular hydrocephalus: a single-institutional experience. Childs Nerv Syst. 2012;28(12):2101–2107. doi: 10.1007/s00381-012-1887-5. [DOI] [PubMed] [Google Scholar]
- [15].MacKenzie SH, Clark AC. Death by caspase dimerization. Adv Exp Med Biol. 2012;747:55–73. doi: 10.1007/978-1-4614-3229-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wickman G, Julian L, Olson MF. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012;19:735–742. doi: 10.1038/cdd.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Falschlehner C, Boutros M. Innate immunity: regulation of caspases by IAP-dependent ubiquitylation. EMBO J. 2012;31(12):2750–2752. doi: 10.1038/emboj.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chlastakova I, Liskova M, Kudelova J, et al. Dynamics of caspase-3 activation and inhibition in embryonic micromasses evaluated by a photon-counting chemiluminescence approach. In Vitro Cell Dev Biol Anim. 2012;48(9):545–549. doi: 10.1007/s11626-012-9542-8. [DOI] [PubMed] [Google Scholar]
- [19].Snigdha S, Smith ED, Prieto GA, et al. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci Bull. 2012;28(1):14–24. doi: 10.1007/s12264-012-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Folch J, Alvira D, López-Querol M, et al. Evaluation of transcriptional activity of caspase-3 gene as a marker of acute neurotoxicity in rat cerebellar granular cells. Toxicol In Vitro. 2010;24(2):465–471. doi: 10.1016/j.tiv.2009.09.023. [DOI] [PubMed] [Google Scholar]
- [21].Hwang IK, Ahn JH, Yoo DY, et al. Increased immunoreactivities of cleaved αII-spectrin and cleaved caspase-3 in the aged dog spinal cord. Neurochem Res. 2012;37(3):480–486. doi: 10.1007/s11064-011-0633-9. [DOI] [PubMed] [Google Scholar]
- [22].Liu J, Lu Y, Liang J. A novel fluorescence derivatization method combined with HPLC for determining the activities of endogenous caspase. Analyst. 2012;137(21):5097–5104. doi: 10.1039/c2an35822k. [DOI] [PubMed] [Google Scholar]
- [23].Matsumoto A, Susaki E, Onoyama I, et al. Deregulation of the p57-E2F1-p53 axis results in nonobstructive hydrocephalus and cerebellar malformation in mice. Mol Cell Biol. 2011;31(20):4176–4192. doi: 10.1128/MCB.05370-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Merhar S. Biomarkers in neonatal posthemorrhagic hydrocephalus. Neonatology. 2012;101(1):1–7. doi: 10.1159/000323498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gozes I. Microtubules (tau) as an emerging therapeutic target: NAP (davunetide) Curr Pharm Des. 2011;17(31):3413–3417. doi: 10.2174/138161211798072553. [DOI] [PubMed] [Google Scholar]
- [26].Yan L, Zhou X, Zhou X, et al. Neurotrophic effects of 7,8-dihydroxycoumarin in primary cultured rat cortical neurons. Neurosci Bull. 2012;28(5):493–498. doi: 10.1007/s12264-012-1233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].VanGuilder HD, Farley JA, Yan H, et al. Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline. Neurobiol Dis. 2011;43(1):201–212. doi: 10.1016/j.nbd.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sontag JM, Nunbhakdi-Craig V, White CL, 3rd, et al. The protein phosphatase PP2A/Bα binds to the microtubule-associated proteins Tau and MAP2 at a motif also recognized by the kinase Fyn: implications for tauopathies. J Biol Chem. 2012;287(18):14984–14993. doi: 10.1074/jbc.M111.338681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rodríguez-Vázquez J, Camacho-Arroyo I, Velázquez-Moctezuma J. Differential impact of REM sleep deprivation on cytoskeletal proteins of brain regions involved in sleep regulation. Neuropsychobiology. 2012;65(3):161–167. doi: 10.1159/000330010. [DOI] [PubMed] [Google Scholar]
- [30].Cao MH, Ji FT, Liu L. Expression changes of parvalbumin and microtubule-associated protein 2 induced by chronic constriction injury in rat dorsal root ganglia. Chin Med J (Engl) 2011;124(14):2184–2190. [PubMed] [Google Scholar]
- [31].Konar A, Shah N, Singh R, et al. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One. 2011;6(11):e27265. doi: 10.1371/journal.pone.0027265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Huang S, Zhao F, Zhao Z, et al. Effect of erythropoietin (EPO) on plasticity of nervous synapse in CA1 region of hippocampal of vascular dementia (VaD) rats. Afr J Pharm and Pharmacol. 2012;6(15):1111–1117. [Google Scholar]
- [33].Zhou Q, Zhang Q, Zhao X, et al. Cortical electrical stimulation alone enhances functional recovery and dendritic structures after focal cerebral ischemia in rats. Brain Res. 2010;22(1311):148–157. doi: 10.1016/j.brainres.2009.11.022. [DOI] [PubMed] [Google Scholar]
- [34].Xiao Z, Lin L, Liu Z, et al. Potential therapeutic effects of curcumin: relationship to microtubule-associated proteins 2 in Aβ1-42 insult. Brain Res. 2010;18(1361):115–123. doi: 10.1016/j.brainres.2010.09.019. [DOI] [PubMed] [Google Scholar]
- [35].Céspedes-Rubio A, Jurado FW, Cardona-Gómez GP. p120 catenin/αN-catenin are molecular targets in the neuroprotection and neuronal plasticity mediated by atorvastatin after focal cerebral ischemia. J Neurosci Res. 2010;88(16):3621–3634. doi: 10.1002/jnr.22511. [DOI] [PubMed] [Google Scholar]
- [36].Lee JH, Jang S, Jeong HS, et al. Effects of sphingosine-1-phosphate on neural differentiation and neurite outgrowth in neuroblastoma cells. Chonnam Med J. 2011;47(1):27–30. doi: 10.4068/cmj.2011.47.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yan XX, Cai Y, Zhang XM, et al. BACE1 elevation is associated with aberrant limbic axonal sprouting in epileptic CD1 mice. Exp Neurol. 2012;235(1):228–237. doi: 10.1016/j.expneurol.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Basar S, Uhlenhut K, Högger P, et al. Analgesic and antiinflammatory activity of Morinda citrifolia L. (Noni) fruit. Phytother Res. 2010;24(1):38–42. doi: 10.1002/ptr.2863. [DOI] [PubMed] [Google Scholar]
- [39].Singh DR, Singh S, Salim KM, et al. Estimation of phytochemicals and antioxidant activity of underutilized fruits of Andaman Islands (India) Int J Food Sci Nutr. 2012;63(4):446–452. doi: 10.3109/09637486.2011.634788. [DOI] [PubMed] [Google Scholar]
- [40].Piaru SP, Mahmud R, Abdul Majid AM, et al. Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morinda citrifolia. Asian Pac J Trop Med. 2012;5(4):294–298. doi: 10.1016/S1995-7645(12)60042-X. [DOI] [PubMed] [Google Scholar]
- [41].Kumar DJ, Santhi RJ. Antioxidant and cytotoxic effects of hexane extract of Morinda pubescens leaves in human liver cancer cell line. Asian Pac J Trop Med. 2012;5(5):362–366. doi: 10.1016/S1995-7645(12)60060-1. [DOI] [PubMed] [Google Scholar]
- [42].Lin YL, Chou CH, Yang DJ, et al. Hypolipidemic and antioxidative effects of noni (Morinda citrifolia L.) juice on high-fat/cholesterol-dietary hamsters. Plant Foods Hum Nutr. 2012;67(3):294–302. doi: 10.1007/s11130-012-0309-x. [DOI] [PubMed] [Google Scholar]
- [43].Wang MY, Peng L, Weidenbacher-Hoper V, et al. Noni juice improves serum lipid profiles and other risk markers in cigarette smokers. ScientificWorldJournal 2012. 2012 doi: 10.1100/2012/594657. 594657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Dussossoy E, Brat P, Bony E, et al. Characterization, anti-oxidative and anti-inflammatory effects of Costa Rican noni juice (Morinda citrifolia L.) J Ethnopharmacol. 2011;133(1):108–115. doi: 10.1016/j.jep.2010.08.063. [DOI] [PubMed] [Google Scholar]
- [45].Nualsanit T, Rojanapanthu P, Gritsanapan W, et al. Damnacanthal-induced anti-inflammation is associated with inhibition of NF-κB activity. Inflamm Allergy Drug Targets. 2011;10(6):455–463. doi: 10.2174/187152811798104908. [DOI] [PubMed] [Google Scholar]
- [46].Fox C, Crugel M, Maidment I, et al. Efficacy of memantine for agitation in Alzheimer's dementia: a randomised double-blind placebo controlled trial. PLoS One. 2012;7(5):e35185. doi: 10.1371/journal.pone.0035185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yanagisawa K. Strategy and perspectives for development of Alzheimer disease-modifying drugs. Seishin Shinkeigaku Zasshi. 2012;114(3):262–267. [PubMed] [Google Scholar]
- [48].Gu Z, Nakamura T, Lipton SA. Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol Neurobiol. 2010;41(2-3):55–72. doi: 10.1007/s12035-010-8113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Borre Y, Bosman E, Lemstra S, et al. Memantine partly rescues behavioral and cognitive deficits in an animal model of neurodegeneration. Neuropharmacology. 2012;62(5-6):2010–2017. doi: 10.1016/j.neuropharm.2011.12.034. [DOI] [PubMed] [Google Scholar]
- [50].Filippidis AS, Kalani MY, Rekate HL. Hydrocephalus and aquaporins: lessons learned from the bench. Childs Nerv Syst. 2011;27(1):27–33. doi: 10.1007/s00381-010-1227-6. [DOI] [PubMed] [Google Scholar]
- [51].Reddy GK, Bollam P, Caldito G, et al. Ventriculoperitoneal shunt surgery outcome in adult transition patients with pediatric-onset hydrocephalus. Neurosurgery. 2012;70(2):380–389. doi: 10.1227/NEU.0b013e318231d551. [DOI] [PubMed] [Google Scholar]
- [52].Reddy GK, Shi R, Nanda A, et al. Obstructive hydrocephalus in adultpatients: the Louisiana State University Health Sciences Center- Shreveport experience with ventriculoperitoneal shunts. World Neurosurg. 2011;76(1-2):176–182. doi: 10.1016/j.wneu.2011.01.032. [DOI] [PubMed] [Google Scholar]
- [53].Kosik KS, Duffy LK, Dowling MM, et al. Microtubule-associated protein 2: monoclonal antibodies demonstrate the selective incorporation of certain epitopes into Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1984;81(24):7941–7945. doi: 10.1073/pnas.81.24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Neve RL, Selkoe DJ, Kurnit DM, et al. A cDNA for a human microtubule associated protein 2 epitope in the Alzheimer neurofibrillary tangle. Brain Res. 1986;387(2):193–196. doi: 10.1016/0169-328x(86)90011-2. [DOI] [PubMed] [Google Scholar]
- [55].Bancher C, Lassmann H, Budka H, et al. Neurofibrillary tangles in Alzheimer's disease and progressive supranuclear palsy: antigenic similarities and differences. Microtubule-associated protein tau antigenicity is prominent in all types of tangles. Acta Neuropathol. 1987;74(1):39–46. doi: 10.1007/BF00688336. [DOI] [PubMed] [Google Scholar]
- [56].Yen SH, Dickson DW, Crowe A, et al. Alzheimer's neurofibrillary tangles contain unique epitopes and epitopes in common with the heat-stable microtubule associated proteins tau and MAP2. Am J Pathol. 1987;126(1):81–91. [PMC free article] [PubMed] [Google Scholar]
- [57].Nukina N, Ihara Y. One of the antigenic determinants of paired helical filaments is related to tau protein. J Biochem. 1986;99:1541–1544. doi: 10.1093/oxfordjournals.jbchem.a135625. [DOI] [PubMed] [Google Scholar]
- [58].Guo Y, Gong HS, Zhang J, et al. Remarkable reduction of MAP2 in the brains of scrapie-infected rodents and human prion disease possibly correlated with the increase of calpain. PLoS One. 2012;7(1):e30163. doi: 10.1371/journal.pone.0030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hsia AY, Masliah E, McConlogue L, et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Favaloro B, Allocati N, Graziano V, et al. Role of apoptosis in disease. Aging (Albany NY) 2012;4(5):330–349. doi: 10.18632/aging.100459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rohn TT. The role of caspases in Alzheimer's disease; potential novel therapeutic opportunities. Apoptosis. 2010;15:1403–1409. doi: 10.1007/s10495-010-0463-2. [DOI] [PubMed] [Google Scholar]
- [62].Castejón OJ. Submicroscopic pathology of human and experimental hydrocephalic cerebral cortex. Folia Neuropathol. 2010;48(3):159–174. [PubMed] [Google Scholar]
- [63].Felderhoff-Mueser U, Buhrer C, Groneck P, et al. Soluble Fas (CD95/Apo-1), soluble Fas ligand, and activated caspase 3 in the cerebrospinal fluid of infants with posthemorrhagic and nonhemorrhagic hydrocephalus. Pediatr Res. 2003;54(5):659–664. doi: 10.1203/01.PDR.0000084114.83724.65. [DOI] [PubMed] [Google Scholar]
- [64].Klyubin I, Wang Q, Reed MN, et al. Protection against Abeta-mediated rapid disruption of synaptic plasticity and memory by memantine. Neurobiol Aging. 2011;32(4):614–623. doi: 10.1016/j.neurobiolaging.2009.04.005. [DOI] [PubMed] [Google Scholar]
- [65].Abdel-Aal RA, Assi AA, Kostandy BB. Memantine prevents aluminum-induced cognitive deficit in rats. Behav Brain Res. 2011;225(1):31–38. doi: 10.1016/j.bbr.2011.06.031. [DOI] [PubMed] [Google Scholar]
- [66].Bordji K, Becerril-Ortega J, Nicole O, et al. Activation of extrasynaptic, but not synaptic, NMDA receptors modifies amyloid precursor protein expression pattern and increases amyloid-ss production. J Neurosci. 2010;30:15927–15942. doi: 10.1523/JNEUROSCI.3021-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Martinez-Coria H, Green KN, Billings LM, et al. Memantine improves cognition and reduces Alzheimer's-like neuropathology in transgenic mice. Am J Pathol. 2010;176(2):870–880. doi: 10.2353/ajpath.2010.090452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Milnerwood AJ, Gladding CM, Pouladi MA, et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington's disease mice. Neuron. 2010;65(2):178–190. doi: 10.1016/j.neuron.2010.01.008. [DOI] [PubMed] [Google Scholar]
- [69].Milnerwood AJ, Raymond LA. Early synaptic pathophysiology in neurodegeneration: insights from Huntington's disease. Trends Neurosci. 2010;33(11):513–523. doi: 10.1016/j.tins.2010.08.002. [DOI] [PubMed] [Google Scholar]
- [70].Kaufman AM, Milnerwood AJ, Sepers MD, et al. Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci. 2012;32(12):3992–4003. doi: 10.1523/JNEUROSCI.4129-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sultzer DL, Melrose RJ, Harwood DG, et al. Effect of memantine treatment on regional cortical metabolism in Alzheimer's disease. Am J Geriatr Psychiatry. 2010;18(7):606–614. doi: 10.1097/JGP.0b013e3181ca3a4e. [DOI] [PubMed] [Google Scholar]
- [72].Potterat O, Felten RV, Dalsgaard PW, et al. Identification of TLC markers and quantification by HPLC-MS of various constituents in noni fruit powder and commercial noni-derived products. J Agric Food Chem. 2007;55(18):7489–7494. doi: 10.1021/jf071359a. [DOI] [PubMed] [Google Scholar]
- [73].Su BN, Pawlus AD, Jung HA, et al. Chemical constituents of the fruits of Morinda citrifolia (Noni) and their antioxidant activity. J Nat Prod. 2005;68(4):592–595. doi: 10.1021/np0495985. [DOI] [PubMed] [Google Scholar]
- [74].Hai J, Su SH, Lin Q, et al. Cognitive impairment and changes of neuronal plasticity in rats of chronic cerebral hypoperfusion associated with cerebral arteriovenous malformations. Acta Neurol Belg. 2010;110(2):180–185. [PubMed] [Google Scholar]
- [75].Urban I, Richard PA. Springfield, IL: Charles C Thomas; 1972. A Stereotaxic Atlas of the New Zealand Rabbit's Brain. [Google Scholar]
- [76].Ceylan S, Anik I, Koc K, et al. Microsurgical anatomy of membranous layers of the pituitary gland and the expression of extracellular matrix collagenous proteins. Acta Neurochir (Wien) 2011;153(12):2435–2443. doi: 10.1007/s00701-011-1182-3. [DOI] [PubMed] [Google Scholar]


