Figure 3.
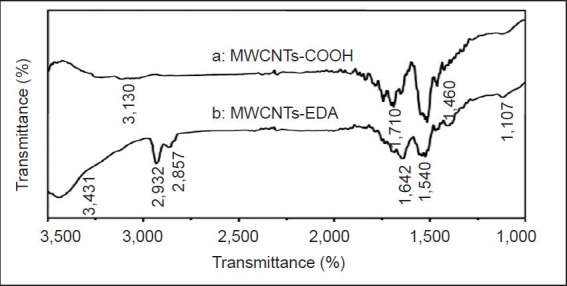
Fourier transform infrared spectroscopy of the MWCNTs- COOH (curve a) and MWCNTs-EDA (curve b).
(A) Infrared spectroscopic characterization of carboxylic CNTs shows that MWCNT surfaces have functional groups, including carbonyl groups at 710/cm and carboxyl groups at 3,130 and 1460/cm. (B) A shift of the band center from 1,710/cm to 1,642/cm indicates that the carboxylic acid groups in MWCNTs completely reacted with the amino groups in EDA to form amide groups. Infrared absorption of the free amino groups in EDA appears at 3,431/cm because of the stretching mode of the N-H bond and at 1,540 and 1,107/cm because of the in-plane vibration mode of the N-H and C-N bonds. The bands at 2,932 and 2,857/cm result from the C-H stretch vibration. MWCNTs: Multi-walled carbon nanotubes; EDA: ethylenediamine.
