Abstract
Ghrelin, a brain-gut peptide that induces anxiety and other abnormal emotions, contributes to the effects of insomnia on emotional behavior. In contrast, the traditional Chinese Medicine remedy Wen Dan Tang reduces insomnia-related anxiety, which may perhaps correspond to changes in the brain-gut axis. This suggests a possible relationship between Wen Dan Tang's pharmacological mechanism and the brain-gut axis. Based on this hypothesis, a sleep-deprived rat model was induced and Wen Dan Tang was administered using oral gavage during model establishment. Wen Dan Tang significantly reduced insomnia-related anxiety and prevented Ghrelin level decreases following sleep deprivation, especially in the hypothalamus. Increased expression of Ghrelin receptor mRNA in the hypothalamus was also observed, suggesting that reduced anxiety may be a result of Wen Dan Tang's regulation of Ghrelin-Ghrelin receptors.
Keywords: nerve regeneration, Chinese Medicine, Wen Dan Tang, sleep deprivation, anxiety, Ghrel in, Ghrelin receptor, the National Natural Science Youth Foundation in China, neural regeneration
Introduction
Insomnia has a high and increasing prevalence in many countries. In this regard, insomnia affects approximately 33% of people in the United States, 4–22% in Europe, and 18–23% in Japan[1]. Results from a recent global sleep survey in China released by the Chinese Society of Psychiatry in 2003 revealed that 42.5% of people in China suffer from insomnia[2]. A large amount of clinical epidemiology and experimental data show that insomnia is closely related to other health problems such as obesity[3], hypertension[4], hyperlipidemia[5], diabetes[5], depression[6], and Alzheimer's disease[7]. With increased fatigue, a series of physiological, psychological, and even behavioral changes occur. These in turn may affect emotions, learning and memory, and immunity[8,9]. A study by Neckelmann and colleagues[10] reported that patients suffering from insomnia are usually also troubled by other emotional disorders, especially depression, anxiety, nervousness, and irritability. Another study by Ma et al.[11] revealed that amongst the participants in their survey, 31% of primary insomnia patients suffer from moderate to severe depression. In this same study, the relationship was even more prominent between insomnia and anxiety, with 54% of primary insomnia patients having both conditions concurrently. The problems that insomnia and its related complications cause are deeply rooted in our modern daily society, thus making sleep and its related neurobiology a major focus of current research.
Previous researches have shown that one of the core disorders produced in the above-mentioned problems is a brain-gut axis imbalance. Reduced sleeping hours trigger deregulation of neuroendocrine networks connecting the central nervous system and the gastrointestinal tract, resulting in several physiological changes such as decreased blood glucose tolerance and insulin sensitivity[12] and increased cortisol[13] and amyloid β protein levels[14]. These changes are strongly associated with fluctuations of the neuropeptide orexin, which is in turn regulated by the brain-gut peptide leptin/Ghrelin ratio[8,15,16,17]. Hence, our study was designed to investigate brain-gut axis mechanisms involved in the relationship between anxiety and insomnia.
Wen Dan Tang, taken from 12th-century Chinese Medicine classic Treatise on Diseases, Patterns, and Prescriptions Related to Unification of the Three Etiologies, is a traditional remedy that has been reported to show significant therapeutic effects during insomnia treatment. The original prescription, however, dates back as far as the Northern and Southern Dynasties (from 420 A.D. to 589 A.D.), where it was first named in Yao Seng Yuan's work Collection of Effective Prescriptions. Wen Dan Tang was designed to induce “Gallbladder Warming”, as it originally targeted diseases with “gallbladder cold” syndrome. It was later adjusted when the concept of “gallbladder cold” expanded to “gallbladder depression and phlegm obstruction”, leading to our present-day Wen Dan Tang that treats all syndromes related to “Qi depression producing fluids, and fluids fighting with Qi”. From a traditional Chinese medicine perspective, phlegm follows the movement of Qi, reaching everywhere or lingering in the middle energizer, obstructing organ systems such as the liver, gallbladder, spleen, and stomach. It may also cloud the heart and disrupt the heart spirit, or permeate the triple energizer. These syndromes are coherent with the extensive neuroendocrine system complications caused by insufficient sleep. Wen Dan Tang's extensive use in treating both neuropsychiatric and digestive system diseases[18,19,20] corresponds with functions of the brain-gut axis. Therefore, we have chosen the Wen Dan Tang for intervention treatment based on prediction of its therapeutic effects in insomnia-related anxiety being related to the brain-gut axis. Researches thus far have shown that Wen Dan Tang can decrease 5-hydroxytryptamine levels[21] in insomnia rats, normalize 5-hydroxyindoleaceticacid levels[22], decrease brain norepinephrine levels in insomnia-model rats, increase interleukin and tumor necrosis factor[23] amounts to standard levels or higher, increase γ-aminobutyric acid[24] levels, increase cholecystokinin 8[25] immunoactivity, reduce damage to and improve functions of neurons in the thalamic nuclei, and provide neuroprotective effects[14]. However, few studies have examined how Wen Dan Tang affects insomnia-related anxiety.
Ghrelin, an endogenous ligand of the growth hormone secretagogue receptor, is a relatively less well-studied brain-gut peptide. It consists of 28 amino acid residues and is widely distributed in the body[26], existing in both the gastrointestinal tract and central nervous system. Specific binding of Ghrelin to the growth hormone secretagogue receptor is important for many biological activities, such as regulation of neuroendocrine axis activity[27], promoting appetite[28], improving learning and memory[29] and sleep quality[30], neuroprotective effects for the brain[31], and affecting the cardiovascular[32] and immune[33] systems. Recent research has also shown that Ghrelin can induce stress, anxiety, and depression. For example, Asakawa et al.[34] showed that when Ghrelin injection into the ventricular system and peritoneal cavity of mice induced obvious anxiety-like behavior in an elevated plus maze test. Kristensson et al.[35] found that acute psychological stress can activate Ghrelin secretion. As a brain-gut peptide, Ghrelin affects the entire brain-gut axis. Because of its obvious effects on insomnia-related anxiety, we focused on Ghrelin as a stepping stone to draw attention to the effects that brain-gut axis has on insomnia complications.
In our experiment, we examined the long-acting benzodiazepine diazepam as our positive control. It is a central nervous system depressant with anxiolytic, sedative, hypnotic, anticonvulsant, antiepileptic, and central-acting muscle relaxant properties. Diazepam also has high selectivity in its sedative, hypnotic, and anxiolytic effects. It stimulates γ-aminobutyric acid receptors in the ascending reticular activating system, increases γ-aminobutyric acid inhibition of the central nervous system, and enhances stimulation of the reticular formation in the brainstem, which leads to inhibition and obstruction of cortical and mesolimbic arousal.
Blood serum, prefrontal cortex, and hypothalamus were selected as the areas examined in our experiment. The hypothalamus is widely recognized as the “sleep center” and “awakening center" and directly regulates the body's sleep cycle. Prefrontal cortex, on the other hand, is closely related to affective states. Blood serum is the medium for brain-gut peptide transportation. We generated a sleep deprivation rat model, and orally administered Wen Dan Tang. Our studies provide evidence of whether the therapeutic effects of Wen Dan Tang for insomnia-related anxiety are related to the brain-gut axis, and provide a basis for future research on insomnia-caused brain-gut axis imbalances and neuroendocrine disorders.
Results
Quantitative analysis of experimental animals
A total of 48 Sprague-Dawley rats were equally and randomly distributed into a (1) control group: same raising period, no intervention, (2) model group: sleep deprived using a multi-platform sleep deprivation technique, (3) diazepam group: oral gavage using diazepam solution during model establishment, and (4) Wen Dan Tang group: Wen Dan Tang administered by oral gavage during model establishment. None of the experimental animals died. All 48 rats were included in the final analysis, and 12 rats from each group were subjected to behavioral analysis to provide a sufficient sample size for a valid behavioral analysis. Five samples were drawn from each group, and subjected to enzyme-linked immunosorbent assay (ELISA), immunohistochemistry and PCR tests, to provide a sufficient sample size for a valid experimental result.
Wen Dan Tang decoction did not affect body weight in sleep-deprived rats
No significant difference was observed for the body weight of rats in each group before sleep deprivation (P > 0.05). Compared with the control group, rats in the other three groups had significant weight loss after 7 days of sleep deprivation (P < 0.01), and diazepam or Wen Dan Tang did not affect weight loss in sleep-deprived rats (P > 0.05; Table 1).
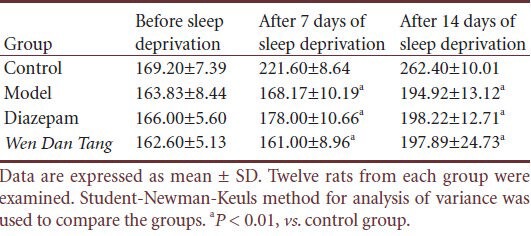
Table 1.
Effects of Wen Dan Tang on body weight (g) in sleep-deprived rats
Wen Dan Tang decoction normalizes behavior in sleep-deprived rats
Grid crossing
No significant difference was observed for mean grid crossing values between rats of different groups before sleep deprivation (P > 0.05). Compared with rats in the control group, grid crossing value increased after sleep deprivation (P < 0.01). However, grid crossing values decreased in Wen Dan Tang-administered rats (after 14 days of sleep deprivation; P < 0.01), with values lower than that of the diazepam group (after 14 days of sleep deprivation; P < 0.01; Table 2).
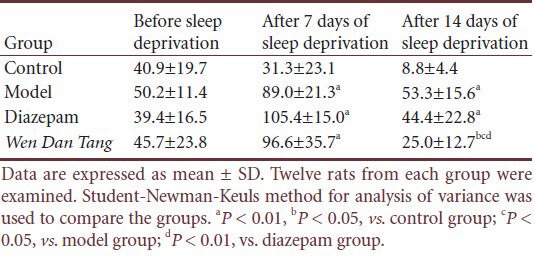
Table 2.
Effects of Wen Dan Tang on grid crossing values in sleepdeprived rats (times/5 minutes)
Standing frequency
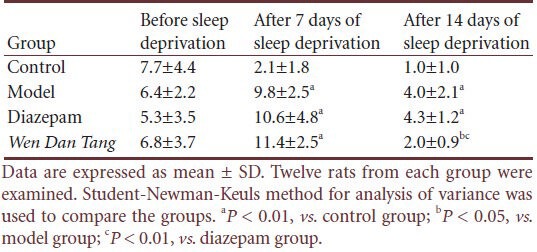
No significant difference was observed between rats of different groups before sleep deprivation in standing frequency (P > 0.05). Compared with the control group, standing frequency increased after sleep deprivation (P < 0.01). However, Wen Dan Tang-administered rats displayed a lower standing frequency (after 14 days of sleep deprivation; P < 0.05), with values below that of the diazepam group (after 14 days of sleep deprivation; P < 0.01; Table 3).
Table 3.
Effects of Wen Dan Tang on standing frequency in sleep-deprived rats (times/5 minutes)
Effects of Wen Dan Tang on Ghrelin expression and Ghrelin receptor mRNA levels in sleep-deprived rats
Blood serum Ghrelin
Results from ELISA analysis showed that no significant difference was detected between blood serum Ghrelin levels in all four groups. Ghrelin levels in control group rats were 937.30 ± 89.47 ng/L, and 880.31 ± 33.86 ng/L in rats after 14 days’ sleep deprivation. Ghrelin levels in the Wen Dan Tang and diazepam groups were 836.07 ± 116.90 and 783.76 ± 168.89 ng/L, respectively.
Ghrelin levels in prefrontal cortex and hypothalamus
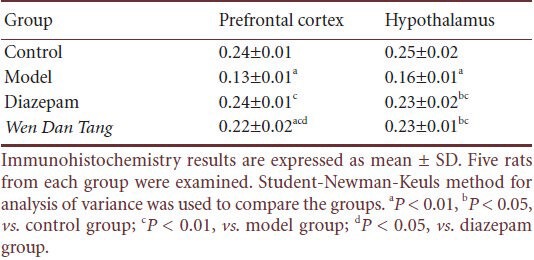
Compared with rats in the control group, immunohistochemistry tests showed that Ghrelin expression significantly decreased in the hypothalamus and prefrontal cortex after 14 days of sleep deprivation (P < 0.01). The Wen Dan Tang group showed increased Ghrelin expression in both the hypothalamus and prefrontal cortex (P < 0.01). No significant difference was observed between Ghrelin levels in the Wen Dan Tang and diazepam groups in the hypothalamus (P > 0.05), but Ghrelin expression in the Wen Dan Tang group was significantly lower than in the diazepam group in the prefrontal cortex (P > 0.05; Figure 1, Table 4).
Figure 1.
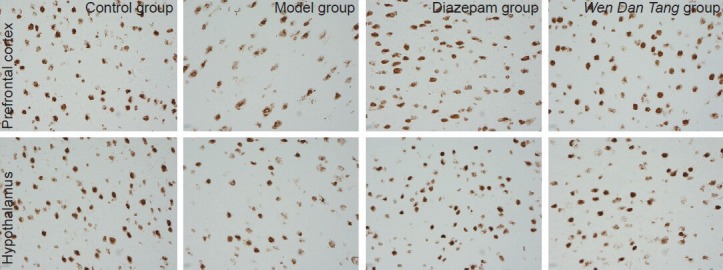
Effects of Wen Dan Tang on Ghrelin expression in the prefrontal cortex and hypothalamus (immunohistochemistry staining, × 400).
In the prefrontal cortex and hypothalamus, positive reactions for Ghrelin were focused inside the cells. Immunoreactive products were brown, and small brown granules can be seen in the cytoplasm of positive cells. No specific color changes were visible in the negative control. In the control group, both the prefrontal cortex and hypothalamus displayed a large amount of positive cells, with deeper and more even staining in the cytoplasm. Cell shape was normal. In the model group, the amount of positive cells in the prefrontal cortex and hypothalamus was less, staining was light, and the cytoplasm was not evenly stained. Cell shape was disrupted, with rupturing in some of the cells, especially in the prefrontal cortex. In the diazepam and Wen Dan Tang groups, large amounts of positive cells were observed in both the prefrontal cortex and hypothalamus. Staining was deep and uniform within the cytoplasm. Cell shape was slightly disordered, and a few cells were ruptured.
Table 4.
Effects of Wen Dan Tang on Ghrelin expression levels in the prefrontal cortex and hypothalamus of 14-day sleep-deprived rats (absorbance)
Ghrelin receptor mRNA levels in the prefrontal cortex and hypothalamus
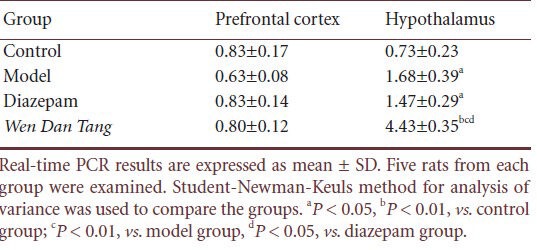
Using real-time PCR, we observed that there were no significant differences in prefrontal cortex Ghrelin receptor mRNA expression between the model, Wen Dan Tang, and diazepam groups after 14 days of sleep deprivation (P > 0.05). Compared with the control group, after 14 days of sleep deprivation, hypothalamus Ghrelin receptor mRNA levels were higher (P < 0.05), whereas no significant differences were observed in the diazepam group. After 14 days’ sleep deprivation, rats administered Wen Dan Tang showed a significant increase in hypothalamus Ghrelin receptor mRNA levels (P < 0.01; Table 5, supplementary Figure 1 online).
Table 5.
Effects of Wen Dan Tang on Ghrelin receptor mRNA levels in the prefrontal cortex and hypothalamus of sleep-deprived rats
Discussion
Open field tests were conducted after 7 and 14 days of sleep deprivation. The open field test can be used to assess general locomotor activity, willingness to explore, and depression- and anxiety-like behavior[36,37]. We measured locomotor activity[38] by grid crossing and standing frequencies. The grid crossing value represents horizontal movement, which is a reflection of the rat's activity level and excitability when placed in an open field box[39]. Standing frequency represents vertical movement, indicating curiosity and the exploratory activity of the rat in a new environment[40]. The excitability of the rats in the 7-day and 14-day model groups was higher than the control group. Considering both psychomotor agitation and a drop in weight, we can determine that sleep deprivation induces anxiety in rats. This is consistent with research by some scholars[8,11,41]. Drug efficacy studies have also shown that Wen Dan Tang significantly improves anxiety induced by sleep deprivation in 14-day sleep-deprived rats, but is ineffective in 7-day rat models. We conclude that the effects of Wen Dan Tang are significantly better than diazepam. Hence, we will continue to study how Wen Dan Tang affects Ghrelin in 14-day sleep-deprived rats.
Sleep deprivation and both drugs had no obvious regulatory effects on blood serum Ghrelin levels. Ghrelin levels in the blood circulation are affected by various factors, such as eating, obesity, insulin secretion, and somatostatin concentrations. It is therefore difficult to exclude the influence of these factors, and blood serum Ghrelin does appear to be a suitable marker of central effects, based on our experiment. Ghrelin expression increases in the prefrontal cortex and hypothalamus after sleep deprivation, and Wen Dan Tang can significantly reduce these effects. Loss of Ghrelin is especially obvious in the hypothalamus. Wen Dan Tang also upregulates Ghrelin receptor mRNA expression in the prefrontal cortex and hypothalamus. However, the different degrees of changes in Ghrelin and Ghrelin receptor mRNA between the prefrontal cortex and hypothalamus is worth considering. We predict that this difference may be related to varying sensitivity in different parts of the brain, the Ghrelin secretion pathway, and delayed Ghrelin-growth hormone secretagogue receptor reactions. There is extensive communication between the prefrontal cortex and hypothalamus, laying a structural basis for stress transmission. The hypothalamus, being the regulatory center for sleep, is directly affected by factors that initiate insomnia, whereas the prefrontal cortex is a downstream target organ that regulates emotions induced by insomnia. Responses in the hypothalamus should thus be quicker and more direct than in the prefrontal cortex. The hypothalamus is the main organ in the central nervous system that synthesizes and secretes Ghrelin. Ghrelin secretion changes occur when the hypothalamus is stimulated, and changes in Ghrelin receptor expression only occur in response to the Ghrelin secretion. Hence, a reaction time lag exists between Ghrelin and Ghrelin receptor responses. In addition, the prefrontal cortex is a downstream organ. This may also affect the latency and degree of the response. Another point to consider would be that Ghrelin receptor mRNA expression may not equate to Ghrelin receptor levels in the cell. Biosynthesis usually requires three steps: transcription, translation, and posttranslational modifications. The regulatory effects of Wen Dan Tang may not necessarily occur only at the transcription stage. Different secretion patterns within the various participants of the biosynthesis process may also contribute to possible inconsistencies in cytoplasm mRNA and peptide content. However, the combination of our results is sufficient to determine that the regulatory mechanism is correlated with the hypothalamus, and the hypothalamus is a brain region worth further study.
Materials and Methods
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was conducted at the Neuroimmunology Laboratory, Beijing University of Chinese Medicine, China from January 2010 to February 2012.
Materials
Animals
A total of 48 healthy adult male Sprague-Dawley rats, aged 6 weeks, weighing 160 ± 20 g, were provided by Vital River Laboratories, Beijing, China (license No. SCXK (Jing) 2011-0001). The rats were housed in a clean animal room with six rats per cage, maintained at 22 ± 2°C and 30–40% humidity. Cages were maintained under a 12-hour light/dark cycle (6:00–18:00). They were allowed free access to food and water, and adaptive feeding was maintained for 3 days. All experimental procedures were conducted in accordance with protocols from the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[42].
Drugs
Grade 1 Tong Ren Tang herbs (consisting of pinellia tuber, bamboo shavings, immature orange fruit, dried tangerine peel, poria, honeyed liquorice root in the ratio of 9:9:9:12:5:5), selected by the Beijing Dong Zhi Men Hospital in China, extracted and concentrated into Wen Dan Tang granules. The recommended adult dosage of Wen Dan Tang granules is two packets per day (each packet consisting of extracts equivalent to that of the following raw herbs: 9 g of pinellia tuber, 9 g of bamboo shavings, 9 g of immature orange fruit, 12 g of dried tangerine peel, 5 g of poria, and 5 g of honeyed liquorice root). Each packet was dissolved in 25 mL of distilled water, and heated until the granules completely dissolved. The solution was used after cooling.
Diazepam (C16H13ClN2O), in tablet form (license No. H11020897), was purchased from Beijing Yimin Pharmaceutical Co., Ltd., Beijing, China. Each tablet of 2.5 mg was dissolved in 50 mL of distilled water and stirred until completely dissolved.
Methods
Sleep-deprived rat models established by a modified multi-platform method
A modified multi-platform sleep deprivation box (as shown in Figure 2) made of plastic, with dimensions of 110 cm length, 60 cm width, and 40 cm height, was constructed. Fifteen transparent Plexiglas platforms, each with a diameter of 6.5 cm and height of 8.0 cm, were fixed to the bottom of the box. Each platform was horizontally and vertically spaced apart by 13 cm and 10 cm, respectively. The box was then filled with water, until the water surface reached 1.0 cm below the platforms. The water was maintained at 22 ± 2°C, and the top of the tank was covered with barb wires. Food and water were hung in the box to allow free feeding. The rats were then placed on the platform, and free movement and feeding were allowed. When a rat enters into paradoxical sleep, its muscles relax and it touches the water by bending its head or it drops into the water. This prevents the rats from entering into a paradoxical sleep state[43]. The rats were placed into the sleep deprivation box every day between 18:00 to 12:00 the next day, and returned to their cages for rest before the next cycle was repeated at 18:00. The rats underwent 18 hours of sleep deprivation on a daily basis, and intermittent paradoxical sleep deprivation continued for 14 days. The water in the sleep deprivation box was replaced every day to ensure sanitary conditions.
Figure 2.
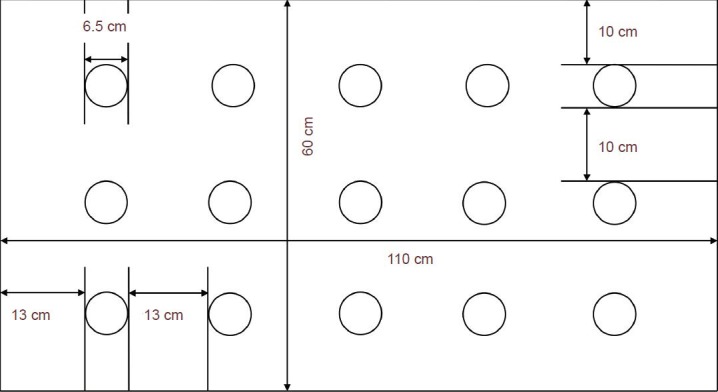
Layout of the custom multi-platform sleep deprivation box used in this study.
Oral gavage treatment
Oral gavage treatment was administered on a daily basis starting from day 1 to day 14 of sleep deprivation. Rats from both the diazepam and Wen Dan Tang groups were given a dose of 1 mL/100 g rat weight[44,45] diazepam solution and Wen Dan Tang, respectively, at 12:00 every day. 1 mL/100 g rat weight of distilled water was administered to rats from the control and model groups.
Measurement of body weight changes in the rats
An electronic scale (from Beijing Feims Technology Development Co., Ltd., Beijing, China) was used to weigh the rats at 8:00 on day 7 and day 14 of sleep deprivation. Drug dosages were adjusted for rats in the diazepam and Wen Dan Tang groups according to their individual weight changes.
Behavioral assessment using the open field test
Behavioral assessment was separately conducted on day 1, day 7, and day 14 of sleep deprivation. The rats were placed into a custom-made, wooden open field box with dimensions of 100 cm length, 100 cm width, and 40 cm height. The interior and floor surfaces were gray, and black lines were drawn to separate the floor into 25 equal-sized 20 cm by 20 cm grids. Grids along the side walls were counted as peripheral grids (16 peripheral square grids), whereas the remaining grids were central grids (nine central square grids). A camera was installed on top of the centermost grid, and the experiments were conducted under quiet, shaded conditions[46]. Before the experiment started, the rats were first placed in the laboratory for 10 minutes to adapt. They were then picked up at 1/3 of the rat tail from its root, and placed gently onto the centermost grid. Synchronized video and timing recordings were collected to observe rat activity within the first 5 minutes. After the rats were removed from the box, a towel moistened by low-concentration ethanol was used to wipe the floor surface to prevent any leftover scents that may disrupt the next rat. The next rat was then placed into the open field box after the ethanol evaporated, and the same cycle was repeated. The activities observed were: (1) total grid crossing value: total number of times the rat placed three or more claws into an adjacent grid; (2) standing frequency: number of times both front limbs were lifted off the floor surface and the rat climbed or rested on the side walls, while using its rear limbs to support its body in a standing position.
Blood serum Ghrelin concentration measured by ELISA
Five rats were randomly selected from all four groups after 14 days of sleep deprivation. Intraperitoneal injection with 10% chloral hydrate (0.35 to 0.40 mL/100 g) was used to anesthetize the rats before they were decapitated for terminal blood withdrawal. The blood was allowed to stand for 20 minutes, followed by centrifugation at 3,000 r/min for 15 minutes. Blood serum was then collected for experiments. The ELISA techniques used followed protocols from the rat growth hormone releasing peptide-Ghelin ELISA Kit, Raybiotech. 100 µL of standard solution and 50 µL of standard diluting solution were first added into wells 1 and 2 of the ELISA microtiter plate. Serial dilution was performed in the subsequent wells with concentrations of 1,200, 800, 400, 200 and 100 ng/L. 40 µL of sample diluting solution and 10 µL of sample were added into sample wells on the microtiter plate. No solution was added into the empty wells. The microtiter plate was then sealed with a closure plate membrane and incubated at 37°C for 30 minutes. The plate was then washed with a mild detergent five times, each time for approximately 30 seconds. 50 µL of ELISA reagent was added into the sample wells. No solution was added into the empty wells. Incubation and washing procedures were repeated as before. 50 µL of both chromogenic reagents A and B were then added into every well. After stirring, the microtiter plate was placed in a dark room at 37°C for 15 minutes. 50 µL of terminating solution was added to terminate the reactions. The detection range was set between 40–1,600 ng/L, whereas the intra and inter coefficient of variation were fixed at lower than 9% and 15%, respectively. Absorbance was tested using a microplate reader (Tecan safire[2], Groedig, Austria) with a wavelength of 450 nm. The actual Ghrelin concentration in each well was then calculated using standard curve linear regression equations for concentration and absorbance.
Immunohistochemical method of Ghrelin expression levels in the rat brain
After 14 days of sleep deprivation, five rats were randomly selected from each group. Intraperitoneal injection with 10% chloral hydrate (0.35 to 0.40 mL/100 g) was used to anesthetize the rats before they were decapitated. The rat brain was then rapidly dissected on ice on top of a clean bench. The entire brain was removed, wrapped up with tin foil and placed into liquid nitrogen for freezing. The frozen rat brain was then put into a cryostat. Each slice was 15 µm thick, and was stored at −20°C for preservation. Brain slices were soaked in 0.3% H2O2 at 37°C for 30 minutes and 1.5% goat serum for 45 minutes, following by incubation with rabbit anti-rat Ghrelin monoclonal antibody (1:50; Beijing Bioss) at 37°C for 1 hour and 4°C overnight. Mouse anti-rabbit antibody (1:200; ANASPEC Company, Fremont, CA, USA) was then used to incubate the sample at 37°C for 45 minutes. The samples were visualized using concentrated 3,3′-diaminobenzidine for 15 minutes, counterstained with hematoxylin, dehydrated through gradient ethanol, and mounted with neutral resin. 0.01 mmol/L PBS (pH 7.4) and deionized water were used to wash the samples between every step. After mounting, the samples were photographed and observed under a Nikon 4500 digital camera (Nikon, Tokyo, Japan) and Nikon E200 microscope (Nikon) under 400 × magnification. Six brain slices from each group were randomly selected for examination. Images of the hypothalamus and prefrontal cortex were taken under the same light intensity, and the Rat Brain Stereotaxic Map[47] was used as a reference for identifying the hypothalamus and prefrontal cortex tissues. NIS-Elements BR 3.1 software (Nikon) was used for image analysis. Mean absorbance values from the positive reactants were used to express the relative amount of protein expression.
Ghrelin receptor mRNA expression levels in rat brain tissues measured by real-time PCR
After 14 days of sleep deprivation, five rats were randomly selected from each group, and intraperitoneally anesthetized with 10% chloral hydrate (0.35 to 0.40 mL/100 g) before they were decapitated. The rat brain was then rapidly dissected on ice on a clean bench. The right prefrontal cortex and hypothalamus were separated, placed into 1.5-mL sterile centrifuge tubes, and frozen in liquid nitrogen before storage in a −80°C refrigerator. Rat brain tissues were taken out of the refrigerator and placed on ice prior to RNA extraction. Trizol (Invitrogen Life Technologies, Carlsbad, CA, USA) was used for one-step extraction of RNA[48]. RNA quantity and concentration were analyzed using an ultraviolet spectrophotometer (NanoDrop® ND-1000, Thermo Fisher, MA, USA) and denaturing agarose gel electrophoresis (DYY-8 steady flow electrophoresis, Shanghai Qite analytical Instrument Corp, Shanghai, China; H6-1 micro-electrophoresis tank, Shanghai Jingyi organic glass Instrument Corp, Shanghai, China). Reverse transcription, aseptically performed on a clean bench, was then applied to synthesize cDNA from the sample's RNA. PCR primers were designed in accordance with protocols from Pubmed Database Genbank Serial Number[49,50] using the Primer 5.0 sequence as a template.
The primer sequences are listed as follows:
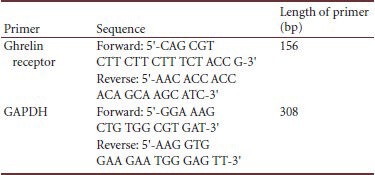
A PCR instrument (ABI 7900 Realtime PCR, ABI, Carlsbad, CA, USA) was used to perform real-time PCR on GADPH cDNA and Ghrelin receptor cDNA. Serial dilution of the DNA template was prepared according to values that will be used in drawing a standard curve. The PCR reaction system was set at 10 µL, of which the template cDNA was 2 µL, dNTP mixture (2.5 mmol/L of dATP, dGTP, dCTP and dTTP) 1.0 µL, 10 × PCR buffer solution (Promega) 1.0 µL, MgCl2 solution (Promega) 0.6 µL, Taq polymerase (Promega) 0.5 units, Sybergreen I final concentration 0.25 × (Invitrogen), 10 µmol/L PCR specific primer 0.4 µL and 2 × ROX Reference Dye (Invitrogen) 0.2 µL. The sample was brought to a final volume of 10 µL with ddH2O. GAPDH and Ghrelin receptor treatment conditions were the same: 95°C denaturation for 3 minutes and 40 cycles of 95°C for 15 seconds, 59°C for 20 seconds, 72°C for 20 seconds, and 82°C for 20 seconds. After amplification, another 40 cycles of 95°C for 15 seconds, 60°C for 20 seconds, 72°C for 20 seconds, and 99°C for 15 seconds, with gradual heating from 72°C to 99°C, was performed (automatic operation – Ramp rate 2%). Measures from various sample concentrations were produced by the instrument according to the pre-planned serial dilution DNA standard curve. The relative expression of Ghrelin receptor mRNA was expressed at a ratio of Ghrelin receptor mRNA to GAPDH gene.
Statistical analysis
All data were expressed as mean ± SD and were analyzed using normal distribution tests and tests of homogeneity of variance between groups with SPSS 17.0 (SPSS, Chicago, IL, USA). A comparison of two samples was performed using the Student-Newman-Keuls method for analysis of variance if the data had a normal distribution and homogeneous variance. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the National Natural Science Youth Foundation in China, No. 81001501; the Natural Science Foundation of Beijing in China, No. 7112071; Beijing University of Chinese Medicine Key Project – Nautical Chinese Medicine in China, No. 522/100604054.
Conflicts of interest: None declared.
Peer review: The article has shown that Wen Dan Tang improves insomnia, possibly through the regulation of endogenous braingut peptides in the brain. This has also further proven the effects of Wen Dan Tang on the brain-gut axis.
Supplementary information: Supplementary data associated with this article can be found in the online version, by visiting www.nrronline.org.
Copyedited by Murnane K, Wang RG, Cong WH, Yu J, Qiu Y, Li CH, Song LP, Liu WJ, Zhao M
References
- [1].Taylor DJ, Mallory LJ, Lichstein KL, et al. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213–218. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- [2].Li YH, Liu Y, Shi T, et al. Middle-aged sleep disorders survey: sleep disorders seriously endanger the quality of life and safety of the middle-aged population. Shoudu Yiyao. 2005;13:6–15. [Google Scholar]
- [3].Hargens TA, Kaleth AS, Edwards ES, et al. Association between sleep disorders, obesity, and exercise: a review. Nat Sci Sleep. 2013;5:27–35. doi: 10.2147/NSS.S34838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Palagini L, Bruno RM, Gemignani A, et al. Sleep loss and hypertension: a systematic review. Curr Pharm Des. 2013;19(13):2409–2419. doi: 10.2174/1381612811319130009. [DOI] [PubMed] [Google Scholar]
- [5].Rööst M, Nilsson P. Sleep disorders--a public health problem. Potential risk factor in the development of type 2 diabetes, hypertension, dyslipidemia and premature aging. Lakartidningen. 2002;99(3):154–157. [PubMed] [Google Scholar]
- [6].Baglioni C, Spiegelhalder K, Nissen C, et al. Clinical implications of the causal relationship between insomnia and depression: how individually tailored treatment of sleeping difficulties could prevent the onset of depression. EPMA J. 2011;2(3):287–293. doi: 10.1007/s13167-011-0079-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Osorio RS, Pirraglia E, Agüera-Ortiz LF, et al. Greater risk of Alzheimer's disease in older adults with insomnia. J Am Geriatr Soc. 2011;59(3):559–562. doi: 10.1111/j.1532-5415.2010.03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Taheri S, Lin L, Austin D, et al. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Alhola P, Polo-Kantola P. Sleep deprivation: Impact on cognitive performance. Neuropsychiatr Dis Treat. 2007;3(5):553–567. [PMC free article] [PubMed] [Google Scholar]
- [10].Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30(7):873–880. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ma T, Chang MH, Tien L, et al. The long-term effect of statins on the risk of new-onset diabetes mellitus in elderly Taiwanese patients with hypertension and dyslipidaemia: a retrospective longitudinal cohort study. Drugs Aging. 2012;29(1):45–51. doi: 10.2165/11597250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [12].Gangwisch JE, Malaspina D, Boden-Albala B, et al. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28(10):1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- [13].Heiser P, Dickhaus B, Schreiber W, et al. White blood cells and cortisol after sleep deprivation and recovery sleep in humans. Eur Arch Psychiatry Clin Neurosci. 2000;250(1):16–23. doi: 10.1007/pl00007534. [DOI] [PubMed] [Google Scholar]
- [14].Liu P, Zhao L, Zhang SL, et al. Modified Wendan Decoction can Attenuate Neurotoxic Action Associated with Alzheimer's Disease. Evid Based Complement Alternat Med. 2009;6(3):325–330. doi: 10.1093/ecam/nem103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kalra SP, Bagnasco M, Otukonyong EE, et al. Rhythmic, reciprocal ghrelin and leptin signaling: new insight in the development of obesity. Regul Pept. 2003;111(1-3):1–11. doi: 10.1016/s0167-0115(02)00305-1. [DOI] [PubMed] [Google Scholar]
- [17].Spiegel K, Tasali E, Penev P, et al. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- [18].Liu YT, Xu R. Treatment of insomnia by Tanreneizu. Jiangxi Zhongyiyao. 2006;1:17. [Google Scholar]
- [19].Wu L, Zhang LP, Ye QL, et al. Antidepressant function of modified wendan decoction. Zhongguo Linchuang Kangfu. 2006;10(23):63. [Google Scholar]
- [20].Wang LY, Li F, Song YH. Application of Wendan Tang in treating neurological disease. Zhongyi Xuebao. 2012;27(8):964–966. [Google Scholar]
- [21].Wang YM, Kong LD, Huang ZQ. Screening of antidepressant fractions of banxia houpu decoction. Zhongguo Zhong Yao Za Zhi. 2002;27(12):932–936. [PubMed] [Google Scholar]
- [22].Yu HT. Clinical observation on treatment of somatic disorder with combination of Xiaoyao Powder and Wendan Decoction. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2006;26(12):1114–1116. [PubMed] [Google Scholar]
- [23].Li JM, Kong LD, Wang YM, et al. Behavioral and biochemical studies on chronic mild stress models in rats treated with a Chinese traditional prescription Banxia-houpu decoction. Life Sci. 2003;74(1):55–73. doi: 10.1016/j.lfs.2003.06.030. [DOI] [PubMed] [Google Scholar]
- [24].Wu XY, Zhao JL, Zhang M, et al. Sedative, hypnotic and anticonvulsant activities of the ethanol fraction from Rhizoma Pinelliae Praeparatum. J Ethnopharmacol. 2011;135(2):325–329. doi: 10.1016/j.jep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- [25].Ma BY, Zhang FL, Zhou JH, et al. Relationship between the amelioration effect of Wendan Tang on sleep and the expression of cholecystokinin 8 in brains of insomnia rats. Zhongguo Linchuang Kangfu. 2006;10(35):45–47. [Google Scholar]
- [26].Cassoni P, Papotti M, Ghè C, et al. Identification, characterization, and biological activity of specific receptors for natural (ghrelin) and synthetic growth hormone secretagogues and analogs in human breast carcinomas and cell lines. J Clin Endocrinol Metab. 2001;86(4):1738–1745. doi: 10.1210/jcem.86.4.7402. [DOI] [PubMed] [Google Scholar]
- [27].Kluge M, Schüssler P, Dresler M, et al. Effects of ghrelin on psychopathology, sleep and secretion of cortisol and growth hormone in patients with major depression. J Psychiatr Res. 2011;45(3):421–426. doi: 10.1016/j.jpsychires.2010.09.002. [DOI] [PubMed] [Google Scholar]
- [28].Dallman MF. Filling the interstices: ghrelin neurons plug several holes in regulation of energy balance. Neuron. 2003;37(4):550–553. doi: 10.1016/s0896-6273(03)00083-7. [DOI] [PubMed] [Google Scholar]
- [29].Carlini VP, Monzón ME, Varas MM, et al. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299(5):739–743. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- [30].Weikel JC, Wichniak A, Ising M, et al. Ghrelin promotes slow-wave sleep in humans. Am J Physiol Endocrinol Metab. 2003;284(2):E407–415. doi: 10.1152/ajpendo.00184.2002. [DOI] [PubMed] [Google Scholar]
- [31].Miao Y, Xia Q, Hou Z, et al. Ghrelin protects cortical neuron against focal ischemia/reperfusion in rats. Biochem Biophys Res Commun. 2007;359(3):795–800. doi: 10.1016/j.bbrc.2007.05.192. [DOI] [PubMed] [Google Scholar]
- [32].Benso A, Broglio F, Marafetti L, et al. Ghrelin and synthetic growth hormone secretagogues are cardioactive molecules with identities and differences. Semin Vasc Med. 2004;4(2):107–114. doi: 10.1055/s-2004-835367. [DOI] [PubMed] [Google Scholar]
- [33].Dixit VD, Yang H, Sun Y, et al. Ghrelin promotes thymopoiesis during aging. J Clin Invest. 2007;117(10):2778–2790. doi: 10.1172/JCI30248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Asakawa A, Inui A, Kaga T, et al. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinology. 2001;74(3):143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- [35].Kristenssson E, Sundqvist M, Astin M, et al. Acute psychological stress raises plasma ghrelin in the rat. Regul Pept. 2006;134(2-3):114–117. doi: 10.1016/j.regpep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [36].Keeney AJ, Hogg S. Behavioural consequences of repeated social defeat in the mouse: preliminary evaluation of a potential animal model of depression. Behav Pharmacol. 1999;10(8):753–764. doi: 10.1097/00008877-199912000-00007. [DOI] [PubMed] [Google Scholar]
- [37].Hendricks DE, Seay B. The open field as a test of emotionality. Behav Res Methods Instrum Comput. 1973;5(1):1–61. [Google Scholar]
- [38].Schloesser RJ, Lehmann M, Martinowich K, et al. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010;15(12):1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Markel AL, Galaktionov YuK, Efimov VM. Factor analysis of rat behavior in an open field test. Neurosci Behav Physiol. 1989;19(4):279–286. doi: 10.1007/BF01236015. [DOI] [PubMed] [Google Scholar]
- [40].Berlin: Springer; 2010. Encyclopedia of Psychopharmacology. [Google Scholar]
- [41].Silva RH, Kameda SR, Carvalho RC, et al. Anxiogenic effect of sleep deprivation in the elevated plus-maze test in mice. Psychopharmacology (Berl) 2004;176(2):115–122. doi: 10.1007/s00213-004-1873-z. [DOI] [PubMed] [Google Scholar]
- [42].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30 [Google Scholar]
- [43].Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav. 2000;68(3):309–316. doi: 10.1016/s0031-9384(99)00181-x. [DOI] [PubMed] [Google Scholar]
- [44].U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER): Guidance for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005 [Google Scholar]
- [45].U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry: food-effect bioavailability and fed bioequivalence studies. 2002 [Google Scholar]
- [46].Gould TD. Hew Jersey: Humana Press; 2011. Mood and anxiety related phenotypes in mice. [Google Scholar]
- [47].Zhuge QC. Beijing: People's Medical Publishing House; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- [48].Yan J, Zhang N, Qi C, et al. One-step real time RT-PCR for detection of microRNAs. Talanta. 2013;110:190–195. doi: 10.1016/j.talanta.2013.02.028. [DOI] [PubMed] [Google Scholar]
- [49].Ghsr growth hormone secretagogue receptor [Rattus norvegicus (Norway rat)]. 2013-05-26. http://www.ncbi.nlm.nih.gov/gene/84022 .
- [50].Gapdh glyceraldehyde-3-phosphate dehydrogenase [Rattus norvegicus (Norway rat)]. 2013-04-27. http://www.ncbi.nlm.nih.gov/gene/24383 .


