Abstract
It is necessary to investigate the longitudinal tensile mechanical characteristics of the middle cerebral artery and the fetal umbilical vein prior to applying fetal umbilical vein transplantation for repair of injured middle cerebral artery. Fifteen fresh fetal umbilical vein specimens and 15 normal human fresh cadaver middle cerebral artery specimens were collected for longitudinal tensile testing at the speed of 0.5 mm/min and at normal human temperature. The results showed that under 16.0 kPa physiological stress, the strain value of fetal umbilical vein specimens was larger, while the maximal stress and elastic modulus values were less than those of middle cerebral artery specimens. Our findings indicate that fetal umbilical vein has good elastic properties and the stress-strain curve of the fetal umbilical vein is similar to that of the middle cerebral artery. Fetal umbilical vein transplantation can, therefore, potentially repair the injured middle cerebral artery.
Keywords: neural regeneration, middle cerebral artery, fetal umbilical vein, tension, stress, strain, elastic modulus, biomechanics, transplantation repair, neuroregeneration
Research Highlights
-
(1)
The stress, strain, elastic modulus and stress-strain curve of fresh fetal umbilical veins and normal human fresh cadaver middle cerebral artery were measured using a longitudinal tensile test.
-
(2)
The stress-strain relationship expression of fetal umbilical veins and middle cerebral artery was established with regression analysis. The mechanical properties of fetal umbilical veins and middle cerebral artery were quantitatively compared and analyzed, and the feasibility of fetal umbilical vein transplantation for middle cerebral artery was investigated using the longitudinal tensile test.
-
(3)
The stress-strain curve of fetal umbilical veins is similar to that of middle cerebral artery, so it can potentially be used to repair injured middle cerebral artery.
INTRODUCTION
Existing extracranial-intracranial bypass and revascularization mainly adopts autologous radial artery and great saphenous vein tissue[1,2]. Although autologous blood vessels have good biocompatibility, autologous vessel transplantation may produce complications, and source vessels may be insufficient. Growing evidence has confirmed that large-caliber artificial vessels can replace human large arteries[3,4], while small-caliber (less than 6 mm) artificial vascular grafts are not satisfactory as the replacement of human small arteries or veins due to thrombosis and vascular occlusion caused by neointimal thickening. The development of arterial and venous substitutes, therefore, is urgently needed[3,4]. Umbilical vein is a biolog-ical vascular substitute, which is characterized by extensive sources, no venous branching, and good flexibility. Increasing numbers of studies have examined the substitutes for small- and medium-sized artery transplantation[5,6,7,8,9,10,11,12,13,14]. Chen et al[15] found that the anastomotic stoma slightly narrowed, lumens slightly widened, and blood flow in fetal umbilical vein was smooth after human fetal umbilical vein was transplanted with canine abdominal aorta for 3.5 years. These findings demonstrate that umbilical vein is suitable for medium-sized artery transplantation. Many scholars have investigated the mechanical properties of the middle cerebral artery[16,17,18,19,20,21,22,23,24,25,26,27,28]. For example, Li et al[29] found that fetal umbilical vein can be used as an alternative to small-caliber arterial grafts through observations of pressure-caliber relationships and elastic modulus of fetal umbilical vein at distal, central, and proximal segments. Sun and colleagues[30] found that the compliance of umbilical veins was the best at 37–40 weeks. Jiao et al[31] showed that the expansion rate of hardening middle cerebral artery wall was increased under a low stress, and the vessel wall may sharply extend with increasing pressure when the stress is greater than 25 kPa. The hardening middle cerebral artery is different from normal middle cerebral artery, having a rigid tube wall and susceptibility to cerebral vascular diseases. However, longitudinal tensile mechanical properties of fresh fetal umbilical veins and normal human fresh cadaver middle cerebral artery remain unclear.
Progressive vascular tension is considered as a good extension mode, and there remains considerable scope and important clinical significance for vascular reconstruction under longitudinal tension[32]. In this study, we observed and analyzed longitudinal tensile mechanical properties of fresh fetal umbilical veins and normal human fresh cadaver middle cerebral artery, in a broader attempt to investigate the feasibility of fetal umbilical vein graft for repairing middle cerebral artery.
RESULTS
The stress-strain curve of fetal umbilical vein and middle cerebral artery
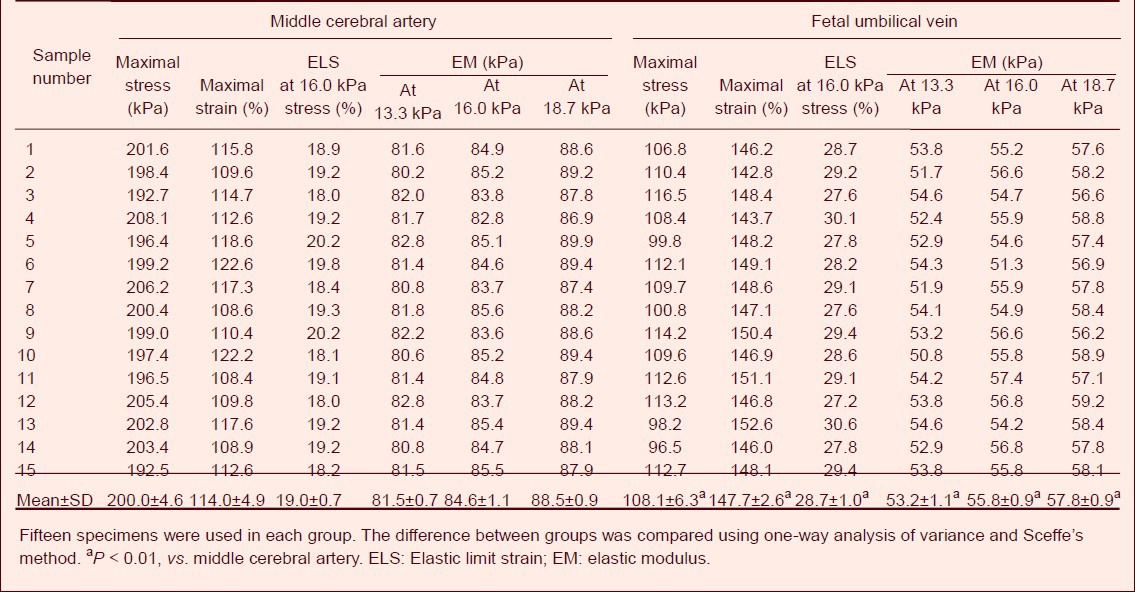
The longitudinal tensile test results showed that when the stress was similar to human systolic pressure of 16.0 kPa, the strain value of the middle cerebral artery specimens was 19%, and that of the fetal umbilical vein specimens was an average 28.7%. When the stress reached 200 kPa and the average strain reached 114%, the middle cerebral artery specimens were inevitably ruptured. These values can therefore be regarded as the maximal stress and strain, respectively, for the middle cerebral artery. When the stress reached 108.0 kPa and the average strain reached 147.7%, the fetal umbilical vein specimens were inevitably ruptured. These values can therefore be regarded as the maximal stress and strain, respectively, for the fetal umbilical vein. Under the same physiological stress, the maximal stress of fetal umbilical vein specimens was decreased (P < 0.01), while the maximal strain was increased compared with the middle cerebral artery specimens (P < 0.01). Fetal umbilical vein specimens showed lower elastic modulus than the middle cerebral artery specimens at 13.3, 16.0 and 18.7 kPa stress (P < 0.01; Table 1).
Table 1.
Longitudinal tensile mechanical indexes of fetal umbilical vein and middle cerebral artery specimens
The tensile data of all the specimens were subject to curve fitting. The stress-strain curve of specimens in two groups showed an exponential relationship when the stress was 16.0 kPa, and the strain of fetal umbilical vein group was higher than the middle cerebral artery group. When the stress exceeded 16.0 kPa, the strain value of specimens in the two groups increased rapidly and the slope of the strain curve also increased as stress increased. The stress-strain correlation was nonlinear, and under continuous forces, the wall fiber gradually extended, prolonged and fractured. The stress-strain curve in the fetal umbilical vein and middle cerebral artery was similar (Figure 1).
Figure 1.
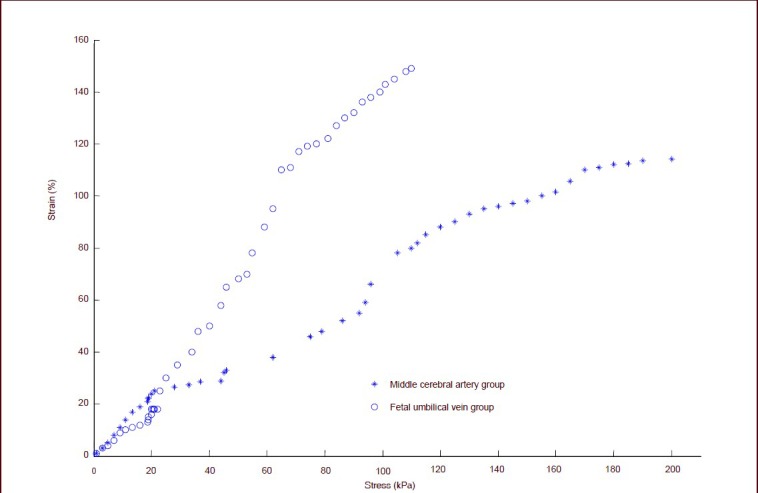
Longitudinal tensile stress-strain curve of fetal umbilical vein and middle cerebral artery specimens.
When the stress is 16.0 kPa, the stress-strain curve of specimens in the two groups shows an exponential relationship. The strain of the fetal umbilical vein was higher than that of the middle cerebral artery. When the stress exceeds 16.0 kPa, the strain of specimens in the two groups increased rapidly and the slope of the strain curve increased as the strain increased, but the stress-strain correlation is nonlinear. The stress-strain curve of fetal umbilical vein and middle cerebral artery is similar.
The stress-strain functional relationship of fetal umbilical vein and middle cerebral artery
The relationship between variables was investigated among the measurement data, and total error was measured. The stress-strain functional relationship was established using a regression analysis method, as follows:
Middle cerebral artery group:
δ(ε) = 0.000 10e5 – 0.006 0e4 + 0.791e3 + 4.98e2
Fetal umbilical vein group:
δ(ε) = –0.000 6e5 + 0.055 9e4 –0.237e3 + 4.018 2e2
DISCUSSION
One-dimensional extension is an important method for comparison of mechanical properties of fetal umbilical vein and middle cerebral artery. However this method may fail and bring about error even in the same species. Discrepancies may be explained by race, gender, age, health condition, occupation and other individual differences.
In this study, we collected fetal umbilical vein specimens from the placenta of healthy women at 38–40 weeks pregnant via caesarean delivery, and harvested middle cerebral artery specimens from healthy adult male cadavers. Both fetal umbilical vein and middle cerebral artery were preserved with the same method, and the tested specimens were sampled from the same site and in the same length. All the specimens were harvested within 24 hours prior to the experiment to maintain freshness. Before the experiment, each specimen in the two groups was preliminarily adjusted to the same speed. All the aforementioned intervention measures were performed to minimize data error.
Experimental and theoretical analysis showed that, tissue reconstruction at longitudinal tension and extension may restore longitudinal stress of blood vessels to normal physiological levels[33,34,35]. Our findings revealed that large deformations occurred when the stress was less than or equal to normal physiological stress values (normal diastolic pressure 16.0 kPa), and the strain disproportionately increased when the stress exceeded 16.0 kPa. This evidence indicates that under loading, the strain value of middle cerebral arterial wall cannot increase with increasing stress. These findings show an exponential change. This result underlines the important contribution to the diagnosis of patients who present with normally performing cerebral vessels and no clinical symptoms when blood pressure increases. Although we only performed longitudinal tensile studies, rather than pressure tests on fetal umbilical vein and middle cerebral artery, we believe that both longitudinal tensile and pressure tests aim to load the stress on the vascular wall. Furthermore the testing speed in this study was very low and the stress was evenly distributed on the vascular wall, so our longitudinal tensile testing results are comparable to the pressure test.
The stress-strain curve is very similar between fetal umbilical vein and middle cerebral artery. The elastic modulus represents the arterial rigidity and elasticity, and the distensibility coefficient is inversely proportional to elastic modulus. The higher the elastic modulus, the lower the distensibility coefficient, and the worse the elasticity[24]. The elastic modulus is the reciprocal of compliance[13]. At the stress of 13.3, 16 and 18.7 kPa, the elastic modulus of fetal umbilical vein specimens was lower than the middle cerebral artery. This evidence indirectly reflects good flexibility and the compliance of fetal umbilical venous wall, which is conducive for the repair of injured middle cerebral artery, as previously reported[12,13,36].
It is worth noting that the middle cerebral artery cannot rupture under normal physiological conditions, although the maximal stress of fetal umbilical vein is less than the middle cerebral artery. The maximum stress of fetal umbilical venous wall is 108.1 kPa, which is 6.75 times higher than normal diastolic blood pressure, so it has no impact on the application of middle cerebral artery injury as an alterative graft material.
In this study, we observed and compared the longitudinal tensile mechanical properties of fresh fetal umbilical veins and normal human fresh cadaver middle cerebral artery. However, there is a large difference between in vitro experiments and in vivo animal experiments. The data we collected were from a limited sample size and was variable due to individual differences seen in biological materials, however, the experimental findings still provide information that can be used in the clinic.
Further investigations of fetal umbilical vein transplantation for middle cerebral artery injury are warranted through animal experiments and preliminary clinical studies.
MATERIALS AND METHODS
Design
A contrast observation regarding biomechanics.
Time and setting
Experiments were performed from August 2011 to January 2012 at the Mechanical Experimental Center of Jilin University in China.
Materials
Fetal umbilical vein was harvested from healthy puerperants aged 25–32 years at 38–40 pregnant weeks scheduled for cesarean section, from China-Japan Friendship Hospital of Jilin University, China. According to the Administrative Regulations on Medical Institutions, issued by the State Council of the People's Republic of China[37], all subjects gave informed consent following being informed of the experimental methods involving fetal umbilical vein prior to experiments. Fetal umbilical vein specimens were preserved in saline (4°C) until use. Middle cerebral artery specimens were obtained from fresh cadavers of normal Chinese adult males, aged 25–33 years, from the Norman Bethune College of Medicine, Jilin University, China. Middle cerebral artery specimens were harvested within 24 hours after death and preserved in saline (4°C).
Methods
Preparation of fetal umbilical vein specimens
After fetal umbilical vein samples were preserved for 1 day, fifteen fetal umbilical vein specimens, 15 mm long, were cut using a S-5 sterile operation knife (Huaian Uniecom Medical Supplies Co., Ltd., Xuyi, Jiangsu Province, China).
Preparation of middle cerebral artery specimens
After middle cerebral artery samples were preserved for 1 day, 15 middle cerebral artery specimens, 15 mm long, were cut for further use.
Tensile test
The thickness and diameter of each specimen were measured using a reading microscope (Changchun Third Optical Instrument Factory, Changchun, Jilin Province, China). Fetal umbilical vein specimens ranged from 0.198–0.206 mm in thickness and 2.92–3.06 mm in diameter, while middle cerebral artery specimens were 0.201–0.212 mm thick, and had a diameter of 3.02–3.10 mm. Two groups of specimens were preliminarily adjusted as previously described[29] and underwent longitudinal tensile tests using a MODEL55100 type automatic control electronic universal testing machine (Changchun Research Institute for Mechanical Science Co., Ltd., Changchun, Jilin Province, China) at the controlled temperature of 36.5 ± 1°C. Specimens were continuously sprayed with physiological saline to maintain tissue humidification. After the test was completed, the maximal load, maximal stress, maximal strain, and elastic modulus were automatically calculated by the computer, and a stress-strain curve was plotted.
Statistical analysis
Measurement data were expressed as mean ± SD and analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA). The difference between groups was compared with one-way analysis of variance and Scheffe's method. P < 0.05 was considered a significant difference. The stress-strain relation expression was established with regression analysis method.
Footnotes
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Ethics Committee, China-Japan Friendship Hospital of Jilin University, China.
(Reviewed by Apricò K, Pack M, Bian LG, Zhang L)
(Edited by Yu J, Yang Y, Li CH, Song LP, Liu WJ, Zhao M)
REFERENCES
- [1].Jeschke MG, Hermanutz V, Wolf SE, et al. Polyurethane vascular prostheses decreases neointimal formation compared with expanded polytetrafluoroethylene. J Vasc Surg. 1999;29(1):168–176. doi: 10.1016/s0741-5214(99)70358-7. [DOI] [PubMed] [Google Scholar]
- [2].Sun YM, Zhang YL, Shi XE, et al. Treatment of giant aneurysms of the cavernous segment of the internal carotid artery with high-flow extracranial and intracranial arteries bypass. Zhongguo Linchuang Shenjing Waike Zazhi. 2011;16(10):7–10. [Google Scholar]
- [3].Xu Y, Zhang JY, Zhang SG. Effectiveness analysis of treatment of femoral-popliteal bypass for artery with atherosclerosis. Yixue yu Zhexue: Linchuang Juece Luntan Ban. 2011;2):54–55. [Google Scholar]
- [4].Sun YG, Xiao F, Huang BT. Treatment of aortic manually with the flap vascular graft for aortic root aneurysm-like dilatation: 18 cases. Guangxi Yixue. 2012;34(3):315–316. [Google Scholar]
- [5].Zhang SQ, Yin CY, Yu JJ. An experimental study on the establishment of internal iliac artery chemotherpeutic conduit by grafting of human umbilical vein. Shiyong Aizheng Zazhi. 1996;11(6):244–246. [Google Scholar]
- [6].Seye CI, Kong Q, Yu N, et al. P2 receptors in atherosclerosis and postangioplasty restenosis. Purinergic Signal. 2007;3(1-2):153–162. doi: 10.1007/s11302-006-9047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Amisten S, Melander O, Wihlborg AK, et al. Increased risk of acute myocardial infarction and elevated levels of C-reactive protein in carriers of the Thr-87 variant of the ATP receptor P2Y11. Eur Heart J. 2007;28(1):13–18. doi: 10.1093/eurheartj/ehl410. [DOI] [PubMed] [Google Scholar]
- [8].Zeng AM, Yao M. Moyamoya disease routine superficial temporal artery: artery bypass grafting surgery with the brain. Zhongguo Wuzhen Xue Zazhi. 2012;12(6):37–43. [Google Scholar]
- [9].Dong WM, Zhang D, Wang S. Cause analysis of delayed intracranial hemorrhage after moyamoya disease of the superficial temporal artery-middle cerebral artery bypass surgery. Zhongguo Shenjing Jingshen Jibing Zazhi. 2010;36(10):597–602. [Google Scholar]
- [10].Stokes L, Surprenant A. Purinergic P2Y2 receptors induce increased MCP-1/CCL2 synthesis and release from rat alveolar and peritoneal macrophages. J Immunol. 2007;179(9):6016–6023. doi: 10.4049/jimmunol.179.9.6016. [DOI] [PubMed] [Google Scholar]
- [11].Jeffree RL, Stoodley MA. STA-MCA bypass for symptomatic carotid occlusion and haemodynamic impairment. J Clin Neurosci. 2009;16(2):226–235. doi: 10.1016/j.jocn.2008.01.022. [DOI] [PubMed] [Google Scholar]
- [12].Seye CI, Yu N, González FA, et al. The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1) J Biol Chem. 2004;279(34):35679–35686. doi: 10.1074/jbc.M401799200. [DOI] [PubMed] [Google Scholar]
- [13].Hayashi K, Handa H, Nagasawa S, et al. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech. 1980;13(2):175–184. doi: 10.1016/0021-9290(80)90191-8. [DOI] [PubMed] [Google Scholar]
- [14].Velcheva I, Antonova N, Damianov P, et al. Common carotid artery hemodynamic factors in patients with cerebral infarctions. Clin Hemorheol Microcirc. 2010;45(2-4):233–238. doi: 10.3233/CH-2010-1306. [DOI] [PubMed] [Google Scholar]
- [15].Chen WP, Guo JG, Ye MF, et al. Experimental study: Umbilical cord vein used as an arterial conduit with a long term follow follow up. Disan Junyi Daxue Xuebao. 1989;10(2):99–101. [Google Scholar]
- [16].Monson KL, Barbaro NM, Manley GT. Biaxial response of passive human cerebral arteries. Ann Biomed Eng. 2008;36(12):2028–2041. doi: 10.1007/s10439-008-9578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schmidt D, Mol A, Odermatt B, et al. Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng. 2006;12(11):3223–3232. doi: 10.1089/ten.2006.12.3223. [DOI] [PubMed] [Google Scholar]
- [18].Wang L, Deng S, Lu Y, et al. Increased inflammation and brain injury after transient focal cerebral ischemia in activating transcription factor 3 knockout mice. Neuroscience. 2012;220:100–108. doi: 10.1016/j.neuroscience.2012.06.010. [DOI] [PubMed] [Google Scholar]
- [19].Ahmad A, Khan MM, Javed H, et al. Edaravone ameliorates oxidative stress associated cholinergic dysfunction and limits apoptotic response following focal cerebral ischemia in rat. Mol Cell Biochem. 2012;367(1-2):215–225. doi: 10.1007/s11010-012-1335-6. [DOI] [PubMed] [Google Scholar]
- [20].Lin YC, Ko TL, Shih YH, et al. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42(7):2045–2053. doi: 10.1161/STROKEAHA.110.603621. [DOI] [PubMed] [Google Scholar]
- [21].Sikkel E, Vandenbussche FP, Oepkes D, et al. Effect of an increase of the hematocrit on middle cerebral artery peak and umbilical vein maximum velocities in anemic fetuses. Fetal Diagn Ther. 2003;18(6):472–478. doi: 10.1159/000073146. [DOI] [PubMed] [Google Scholar]
- [22].Ramón y Cajal CL. Umbilical vein and middle cerebral artery blood flow response to partial occlusion by external compression of the umbilical vein (pressure test) J Matern Fetal Neonatal Med. 2002;12(2):104–111. doi: 10.1080/jmf.12.2.104.111. [DOI] [PubMed] [Google Scholar]
- [23].Clerin V, Nichol JW, Petko M, et al. Tissue engineering of arteries by directed remodeling of intact arterial segments. Tissue Eng. 2003;9(3):461–472. doi: 10.1089/107632703322066642. [DOI] [PubMed] [Google Scholar]
- [24].Davis NP, Han HC, Wayman B, et al. Sustained axial loading lengthens arteries in organ culture. Ann Biomed Eng. 2005;33(7):867–877. doi: 10.1007/s10439-005-3488-x. [DOI] [PubMed] [Google Scholar]
- [25].Han HC, Ku DN, Vito RP. Arterial wall adaptation under elevated longitudinal stretch in organ culture. Ann Biomed Eng. 2003;31(4):403–411. doi: 10.1114/1.1561291. [DOI] [PubMed] [Google Scholar]
- [26].Yu B, Sun CJ, Quan TG, et al. Tensile mechanical properties of middle cerebral artery in an atherosclerosis model. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2010;14(24):4445–4448. [Google Scholar]
- [27].Xu RJ, Liu ZQ, Wang JZ, et al. The mechanical properties of three kinds of human arteries. Shengwu Yixue Gongcheng Xue Zazhi. 1988;5(3):143–148. [Google Scholar]
- [28].Jiao H, Cheng HP, Liu ZJ, et al. The relationship of the elasticity modulus of the middle cerebral artery and the composition of intravascular pressure. Zhongguo Shengwu Yixue Gongcheng Xuebao. 1996;16(1):16–19. [Google Scholar]
- [29].Li WC, Zhang LP, Yu MH, et al. The biomechanical properties of human umbilical cord vein at different segment and its clinical application. Zhongguo Linchuang Jiepou Xue Zazhi. 2003;21(6):627–629. [Google Scholar]
- [30].Sun WQ, Li WC, Wang HQ. Biomechanical studies of the fetal umbilical vein. Yiyong Shengwu Lixue. 2001;16(4):222–230. [Google Scholar]
- [31].Jiao H, Cheng HP, Liu ZJ, et al. Biomechanical properties of normal and atherosclerotic middle cerebral arteries. Shengwu Yixue Gongcheng Xue Zazhi. 1996;13(2):109–112. [Google Scholar]
- [32].Han HC, Ku DN, Vito RP. Arterial wall adaptation under elevated longitudinal stretch in organ culture. Ann Biomed Eng. 2003;31(4):403–411. doi: 10.1114/1.1561291. [DOI] [PubMed] [Google Scholar]
- [33].Jackson ZS, Gotlieb AI, Langille BL. Wall tissue remodeling regulates longitudinal tension in arteries. Circ Res. 2002;90(8):918–925. doi: 10.1161/01.res.0000016481.87703.cc. [DOI] [PubMed] [Google Scholar]
- [34].Humphrey JD, Eberth JF, Dye WW, et al. Fundamental role of axial stress in compensatory adaptations by arteries. J Biomech. 2009;42(1):1–8. doi: 10.1016/j.jbiomech.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gleason RL, Humphrey JD. Effects of a sustained extension on arterial growth and remodeling: a theoretical study. J Biomech. 2005;38(6):1255–1261. doi: 10.1016/j.jbiomech.2004.06.017. [DOI] [PubMed] [Google Scholar]
- [36].Ruiz-Razura A, Williams JL, Jr, Reilly CL, et al. Acute intraoperative arterial elongation: histologic, morphologic, and vascular reactivity studies. J Reconstr Microsurg. 1994;10(6):367–373. doi: 10.1055/s-2007-1006605. [DOI] [PubMed] [Google Scholar]
- [37].State Council of the People's Republic of China. Administrative Regulations on Medical Institution. 1994-09-01 [Google Scholar]


