Abstract
Rapamycin, similar to FK506, can promote neural regeneration in vitro. We assumed that the mechanisms of action of rapamycin and FK506 in promoting peripheral nerve regeneration were similar. This study compared the effects of different concentrations of rapamycin and FK506 on Schwann cells and investigated effects and mechanisms of rapamycin on improving peripheral nerve regeneration. Results demonstrated that the lowest rapamycin concentration (1.53 nmol/L) more significantly promoted Schwann cell migration than the highest FK506 concentration (100μmol/L). Rapamycin promoted the secretion of nerve growth factors and upregulated growth-associated protein 43 expression in Schwann cells, but did not significantly affect Schwann cell proliferation. Therefore, rapamycin has potential application in peripheral nerve regeneration therapy.
Keywords: nerve regeneration, peripheral nerve injury, rapamycin, FK506, Schwann cell, cell migration, nerve growth factor, growth-associated protein 43, NSFC grant, neural regeneration
Introduction
The repair of peripheral nerve injuries is still a challenging task in neurosurgery. In the distal injured nerve, Schwann cells deprived of axonal contact proliferate, upregulate the synthesis and release of a variety of neurotrophic factors and basal lamina components that create an appropriate microenvironment for regenerating axons[1,2]. Autologous nerve grafting is considered a gold standard for repairing large lesions in the peripheral nervous system[3,4,5]. However, the donor site is partially degenerated and nerve grafting only restores limited function. Multiple surgeries are also required. Allografts have the same disadvantages as autografts, but they are also sometimes marked by tissue rejection following an immune response[6].
The FKBP-12-binding ligand, FK506, has been successfully used to stimulate nerve regeneration and prevent the rejection of peripheral nerve allografts[7,8]. FK506 possesses a well-studied neuroregenerative effect, stimulating neurite extension in the presence of nerve growth factor in vitro[9], and enhancing nerve regeneration following nerve crush injury[10] and isografting[11]. However, the use of FK506 to stimulate nerve regeneration is limited because of the risk of renal failure[12] and hypertension[13], and its considerable cost. With long-term allografts, FK506 alone or combined with other drugs reportedly cause life-threatening infections[14,15,16].
Like FK506, rapamycin is an immunosuppressant and FKBP-12-binding ligand, and has a neuroregenerative effect in vitro[17,18,19,20]. Rapamycin has been used in clinical trials to prevent allograft rejection and is approved by the US Food and Drug Administration[13]. Rapamycin has lower nephrotoxicity, lower risk of hypertension, and less neoplastic potential than FK506[21,22]. Rapamycin also plays an important role in composite tissue transplants[23,24]. Rapamycin inhibits the mammalian target of rapamycin, induces autophagy, and reduces the toxicity of polyglutamine expansion in fly and mouse models of Huntington's disease[25,26]. Rapamycin is neuroprotective in multiple models of Parkinson's disease, prevents cognitive deficits and reduces amyloid-β levels. Treatment with 25 nmol/L rapamycin protects neurons from degeneration in neuropathic mouse models by improving peripheral myelin protein 22 processing and increasing the number and length of myelin internodes as well as myelin expression[27,28]. Lagoda et al.[29] found that rapamycin has a neuroprotective effect in rat models of cavernous nerve injury. However, the neuroprotective effects of rapamycin and its other actions have not been comprehensively investigated.
Schwann cells are directly involved in peripheral nerve regeneration[30,31]. Therefore, we used Schwann cells as a model to investigate a drug for improving the efficiency of peripheral nerve regeneration. This study investigated the comparative effects of different concentrations of rapamycin and FK506 on Schwann cells and the possible mechanisms of action of rapamycin.
Results
Effect of different concentrations of rapamycin/FK506 on Schwann cell growth
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) results showed that the viability of Schwann cells in the 1.53 nmol/L–100 μmol/L FK506 group increased from 12 to 60 hours. The viability of Schwann cells in the 400 μmol/L FK506 group decreased with time, suggesting that 400 μmol/L of FK506 was toxic to Schwann cells (Figure 1A). After treatment with different concentrations of rapamycin (from 1.53 nmol/L to 100 μmol/L), cell viability increased (by more than 100% compared with that of the control) after only 36 hours. After 36-hour treatment, rapamycin at 1.53 nmol/L optimally promoted cell viability (P < 0.05), but the effect was independent of dose (Figure 1B). Cell viability under 100 and 400 μmol/L rapamycin decreased with time (lower than 100%, control used as 100%; P < 0.05, vs. control), which indicated that 100 and 400 μmol/L rapamycin was cytotoxic (Figure 1C).
Figure 1.
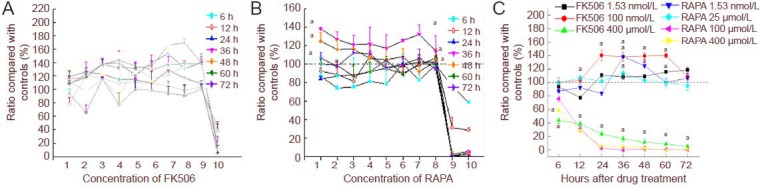
Effects of different concentrations of FK506 and rapamycin (RAPA) on Schwann cell growth at different time points in a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.
Effects of different concentrations of FK506 (A) and RAPA (B) on the growth of Schwann cells at different time points. 1–10 at X-axis: 1.53, 6.10, 24.4, 97.6, 390 nmol/L, 1.56, 6.25, 25, 100, 400 μmol/L; h: hours. (C) Comparison of the effects of FK506 and RAPA on Schwann cell growth. Data are expressed as mean ± SEM from three separate experiments and are compared by one-way analysis of variance and Fisher's post hoc test. aP < 0.05, vs. control.
Effect of FK506 and rapamycin on the cell cycle of Schwann cells
Fluorescence-activated cell sorting analysis revealed that no significant difference in cell cycle distribution ratio and proliferation index of Schwann cells was found among different drug-dose groups (P > 0.05; Figure 2, Table 1).
Figure 2.
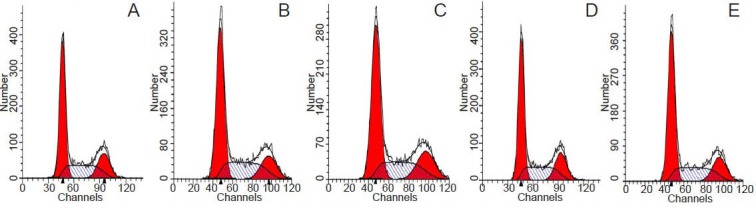
Effect of FK506 and rapamycin on cell cycle (flow cytometry).
(A) Control group; (B, C) FK506 1.53 nmol/L, 100 μmol/L groups; (D, E) rapamycin 25 μmol/L, 1.53 nmol/L groups.
Table 1.
Flow cytometry to determine the effects of FK506 and rapamycin on the cell cycle of Schwann cells
Effect of FK506 and rapamycin on migration of Schwann cells
The different doses of FK506 and rapamycin improved Schwann cell migration speed compared with control (P < 0.05). Rapamycin optimally promoted Schwann cell migration at 1.53 nmol/L compared with the other groups (P < 0.05) and compared with control (P < 0.01; Figure 3).
Figure 3.
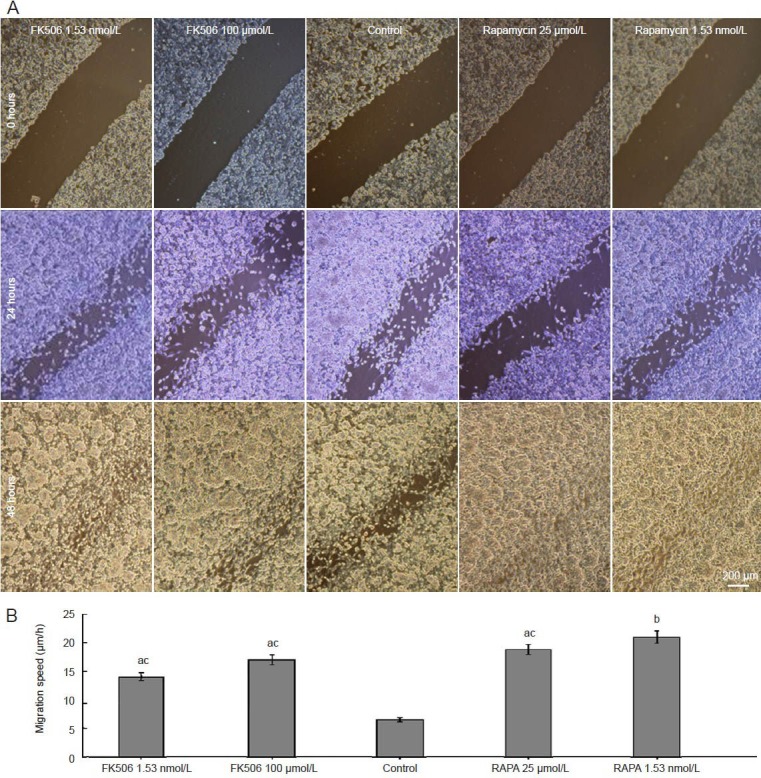
Effect of FK506 or rapamycin (RAPA) on the migration of Schwann cells.
(A) Cell migration assay to determine the effect of ouabain on Schwann cell migration. Bar: 200 μm. (B) The summarized migration speed mea-sured by the wound healing assay. Each column represents mean ± SEM and data were compared by one-way analysis of variance and Fisher's post hoc test. Each experiment was performed in triplicate. aP < 0.05, bP < 0.01, vs. control group; cP < 0.05, vs. RAPA 1.53 nmol/L group.
Effects of FK506/rapamycin on nerve growth factor secretion from Schwann cells
Enzyme linked immunosorbent assay (ELISA) showed that the supernatant of Schwann cells treated with 1.53 nmol/L rapamycin had the highest nerve growth factor concentrations, followed by those treated with 100 μmol/L FK506 (Figure 4).
Figure 4.
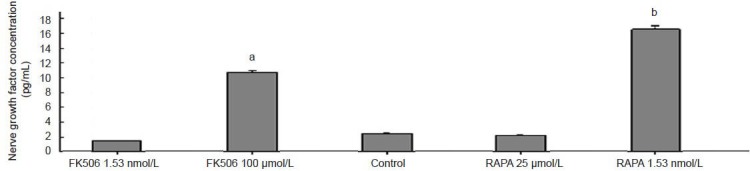
Effects of FK506 or rapamycin (RAPA) on nerve growth factor secretion of Schwann cells according to enzyme linked immunosorbent assay.
Data are expressed as mean ± SEM and are compared by one-way analysis of variance and Fisher's post hoc test. Each experiment was performed in triplicate. aP < 0.05, bP < 0.01, vs. control, FK506 1.53 nmol/L and RAPA 25 μmol/L groups.
Effects of FK506/rapamycin on extracellular signal-regulated kinase 1/2, phosphorylated extracellular signal-regulated kinase 1/2, and growth-associated protein 43 expression
Western blot results showed the expression of extracellular signal-regulated kinases and phosphorylated extracellular signal-regulated kinases did not differ significantly between groups (P > 0.05). The expression of growth-associated protein 43 was significantly downregulated by FK506 (100 μmol/L) and rapamycin (1.53 nmol/L and 25 μmol/L). Rapamycin at 1.53 nmol/L, the lowest dosage among all groups, maximally upregulated growth-associated protein 43 expression (Figure 5).
Figure 5.
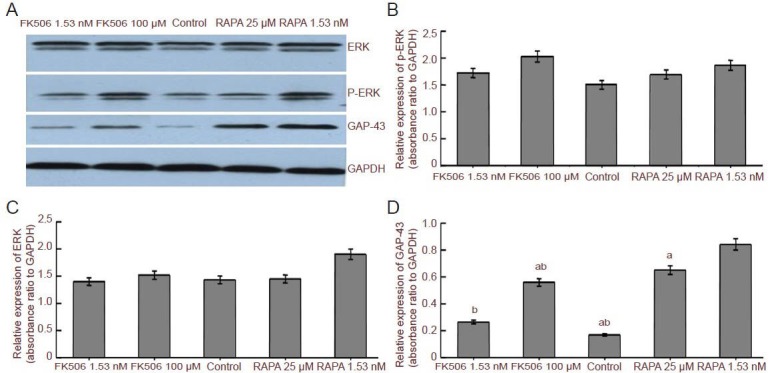
Effect of ERK1/2, P-ERK1/2 and GAP-43 expression in Schwann cells after treatment with FK506/RAPA.
(A) Western blot assay of ERK, p-ERK and GAP-43 in Schwann cells treated by FK506 (1.53 nM, 100 μM) and RAPA (25 μM, 1.53 nM). GAPDH was used as a loading control. (B–D) ERK, p-ERK and GAP-43 expression in Schwann cells treated with FK506 and RAPA. Data are expressed as mean ± SEM and are compared by one-way analysis of variance and Fisher's post hoc test. Each experiment was performed in triplicate. aP < 0.05, vs. control group; bP < 0.05, vs. RAPA 1.53 nM group. M: mol/L; ERK: extracellular signal-regulated kinase; p-ERK: phosphorylated ERK; GAP-43: growth-associated protein 43; RAPA: rapamycin.
Discussion
After peripheral nerve injury, Schwann cells migrate and secrete nerve growth factor and extracellular matrix, which prevents the death of damaged neurons and provides a crucial environment for axonal regeneration. The molecular mechanisms underlying the stimulating effects of FK506 on peripheral nerve regeneration have been reported[32,33]. FKBP-12 binding and calcineurin inhibition can increase the phosphorylation of several substrates, such as growth-associated protein 43, which significantly affects neural plasticity and is highly expressed in regenerative growth cones[34]. Non-immunosuppressant derivatives of FK506 do not inhibit calcineurin, but accelerate nerve regeneration. FK506 and rapamycin, similarly structured immunosuppressants with the same binding proteins, are different and their mechanisms of action are unclear[35]. This study compared the effects of rapamycin and FK506 on Schwann cells and analyzed their possible mechanisms at the cellular level.
Based on literature and preliminary drug screening, we chose drug concentrations ranging from 1.53 nmol/L to 400 μmol/L[11,14,19]. FK506 improved Schwann cell viability within a certain concentration range (24–60 hours), and the effects were not dose-dependent. The optimal FK506 treatment doses were 6.25 and 25 μmol/L. Rapamycin did not reduce the proliferation and migration of fibroblasts[36]. The influence of rapamycin on Schwann cell proliferation and migration remains unknown.
Low-dose rapamycin (1.53 nmol/L) promoted Schwann cell migration at least as much as 100 μmol/L (high-dose) FK506. ELISA indicated that 1.53 nmol/L rapamycin induced Schwann cells to secrete nerve growth factor more significantly than 100 μmol/L FK506. Western blot assay showed that 1.53 nmol/L rapamycin upregulated growth-associated protein 43 expression in Schwann cells more significantly compared with 100 μmol/L FK506. However, this rapamycin concentration did not significantly affect the expression of extracellular signal-regulated kinase 1/2 and phosphorylated extracellular signal-regulated kinase 1/2, which mediated neural cell proliferation and differentiation[37]. Growth-associated protein 43 is a crucial component of axonal and presynaptic terminals and a major phosphoprotein. Growth-associated protein 43 was designated as a GAP, because its synthesis is upregulated during axonal regeneration[38,39].
In damaged facial and sciatic nerves, regeneration is associated with an increase in growth-associated protein 43 mRNA levels in both spinal motor neurons and dorsal root ganglion neurons[40]. It has been suggested that growth-associated protein 43 probably plays a key role in FK-506-induced neuroregeneration, since this drug massively increases the phosphorylation of this major neuronal phosphoprotein implicated in neuronal growth[41,42]. Rapamycin promotes autophagy by inhibiting mammalian target of rapamycin[43]. Rapamycin-sensitive mammalian target of rapamycin activation mediates nerve growth factor-induced cell migration, with the latter being important for nerve regeneration and recovery[44]. Nerve growth factor promotes Schwann cell migration[45,46,47,48]. Growth-associated protein 43 downregulation significantly reduces cell migration[49]. However, whether nerve growth factor secretion promotes cell migration by upregulating growth-associated protein 43 expression still warrants further investigation[50]. The most important form of growth-associated protein 43 for elongation of growth cones is the growth-associated protein phosphorylated by environmental stimuli. Growth-associated protein 43 synthesis in sensory neurons appears to be regulated by nerve growth factor under normal conditions or immediately after axonal injury[51]. Nerve growth factor also changes the intracellular localization of growth-associated protein 43[52,53].
Few animal experiments have demonstrated that rapamycin activates autophagy and improves myelination in explant cultures from neuropathic mice and that it has a neuroprotective effect in a rat model of cavernous nerve injury. Thus, more in vivo tests, especially animal tests on peripheral nerve injury, are warranted to investigate further how rapamycin promotes nerve regeneration.
Low-dose rapamycin (1.53 nmol/L) promotes Schwann cell migration better than high-dose (100 μmol/L) FK506, because rapamycin induces Schwann cells to secrete nerve growth factor and express growth-associated protein 43. Thus, low-dose rapamycin is a potential therapeutic agent, because rapamycin has a lower cost and fewer toxic side effects and rapamycin effectively promotes Schwann cell migration and nerve growth factor secretion. This study demonstrates for the first time a comparison between the effect of rapamycin and FK506 on Schwann cell proliferation, migration and cell cycle, as well as the mechanism by which this effect occurs. Although further clinical trials are necessary to define the risks and benefits of such treatment, particularly in peripheral nerve injury, rapamycin may be an effective and well-tolerated therapeutic option for regenerating peripheral nerves.
Materials and Methods
Design
A controlled cytological study.
Time and setting
Experiments were performed in the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, and Biomedical Materials and Engineering Center, Wuhan University of Technology, China from March 2012 to April 2013.
Materials
Cells
The RSC96 cell line was obtained from the American Type Culture Collection.
Drugs
FK506 and rapamycin were purchased from Sigma-Aldrich (St. Louis, MO, USA) and diluted in a mixture of 90% Dulbecco's modified Eagle's medium (DMEM; Hyclone, Logan, UT, USA) and 10% dimethyl sulfoxide (Sigma-Aldrich) to make a stock solution. Aliquots were stored at −20°C until use.
Methods
Schwann cell culture
The RSC96 cell line was grown separately in 75 cm2 flasks containing 10 mL of DMEM (Hyclone) supplemented with 10% fetal bovine serum (GIBCO-Invitrogen, Grand Island, NY, USA), 1% penicillin, and 1% streptomycin (Hyclone) at 37°C in a fully humidified 5% CO2 atmosphere. Before drug treatments, cells were starved for 24 hours in DMEM containing 1% fetal bovine serum.
Schwann cell proliferation detected by MTT assay
Schwann cell proliferation was evaluated via an MTT assay using 3 × 104 cells in 100 μL of medium per well in a 96-well plate, incubated at 37°C under 5% CO2. Different concentrations of FK506 (1.53, 6.1, 24.4, 97.6, 390 nmol/L and 1.56, 6.25, 25, 100, 400 μmol/L) and of rapamycin (1.53, 6.1, 24.4, 97.6, 390 nmol/L and 1.56, 6.25, 25, 100, 400 μmol/L); Sigme-Aldrich were added to the wells and incubated for 6, 12, 24, 36, 48, 60, and 72 hours. MTT solution (at a final concentration of 5 mg/mL; Sigma-Aldrich) was added to all the wells and incubated at 37°C for 4 hours. The control group was treated with DMEM supplemented with 10% dimethyl sulfoxide and saline. Then, 150 μL of dimethyl sulfoxide was added into each well to dissolve the formazan (MTT metabolic product), and the absorbance of the mixture was read at 570 and 690 nm using a 96-plate reader (Multiskan Spectrum, Thermo Labsystems, Vantaa, Finland). Data were collected from four independent experiments, and each experiment was conducted six times.
Analysis of the Schwann cell cycle using flow cytometry
Schwann cells (1 × 106/mL, 3 mL per well) were seeded in six-well plates. We chose the minimum and maximum doses that improved viability: 1.53 nmol/L and 100 μmol/L for FK506 and 1.53 nmol/L and 25 μmol/L for rapamycin. FK506 or rapamycin (300 μL) was introduced into each well at 1.53 nmol/L and 100 μmol/L. The control group was placed in 300 μL of DMEM supplemented with 10% dimethyl sulfoxide. The plates were then placed in a 5% CO2 incubator at 37°C for 48 hours to observe the cells in the logarithmic phase of growth. Following treatment, the cells were collected by trypsinization and centrifugation, washed with PBS, and fixed with 70% ethanol. The cells were labeled with propidium iodide solution (0.05 mg/mL propidium iodide, 2.00 mg/mL RNase A, 0.01% Triton X-100 in PBS) and incubated for 30 minutes at room temperature in the dark. The cell cycle was closely related to the amount of DNA as DNA content changes with cell cycle change. The cell proliferation index is the total DNA content during the S and G2 phases compared with the total DNA content during the S, G2, and G1 phases. It is one of the most important indexes for cell proliferation. An increase in the percentage of cells in the S phase indicates increased DNA synthesis and cell proliferation[54,55]. The cell cycle (DNA content) was analyzed with a BD flow cytometer (BD Biosciences, San Jose, CA, USA). The results of the cell cycle were analyzed with ModFit 3.0 software (Verity Software House, Topsham, MA, USA).
Schwann cell migration
Schwann cells (5 × 104/mL, 3 mL per well) were seeded in six-well culture plates until a confluent monolayer was obtained. The monolayer was scratched with a pipette tip to create a wound according to a previously described method[56,57]. Each well was then gently washed three times with PBS to remove detached cells, and the cells were cultured in medium containing 2% fetal bovine serum and 1.53 nmol/L to 100 μmol/L FK506 or rapamycin. DMEM supplemented with 10% dimethyl sulfoxide was used as the control. Each experiment was performed with three replicates. Phase contrast images captured the initial distance of the wound created by the pipette tip for each condition. Phase contrast images were taken again after 6, 12, and 24 hours. We counted the cells that invaded the wound in at least seven areas. Each experiment was conducted in triplicate. The influence of the drugs on Schwann cell migration was analyzed in terms of migration speed. Photographs were acquired through a microscope (Olympus IX71, Tokyo, Japan). Briefly, as previously described[58,59], the images acquired for each sample could be further analyzed quantitatively by using computing software. For each image, distances between one side of the scratch and the other could be measured at certain intervals using Image Pro-Plus software (Media Cybernetics, Silver Spring, MD, USA). By comparing the images from time 0 to the last time point (6, 12, 24 hours), the distance of each scratch closure was obtained on the basis of the distances that were measured by software to determine the migration of cells.
Nerve growth factor secreted by Schwann cells was detected using ELISA
Schwann cells (5 × 104/mL, 3 mL per well) were seeded into six-well plates and cultured overnight. FK506 or rapamycin (300 μL) was added to each group in triplicate at final concentrations of 1.53 nmol/L to 100 μmol/L. DMEM supplemented with 10% dimethyl sulfoxide (300 μL) was used as the control. After incubating Schwann cells at 37°C for 48 hours in 5% CO2, the cell supernatant was collected and quantified for the amount of bound nerve growth factor using rabbit anti-nerve growth factor-β monoclonal antibodies (1:1,000; R&D Systems, Minneapolis, MN, USA), with primary antibody-diluted medium according to the manufacturer's instructions. Plates were coated with the “capture” antibodies and maintained overnight at room temperature. Nonspecific binding sites were blocked with 1% bovine serum albumin in PBS. The samples were added at room temperature for 2 hours with gentle agitation. Plates were washed three times, and “detection” antibodies were used to probe the 96-well plates for presence of nerve growth factor. Goat-anti-rabbit IgG labeled with streptavidin-horseradish peroxidase diluted 1:200 in 1% bovine serum albumin-PBS was incubated in the wells for 20 minutes at room temperature. 3,3′,5,5′-Tetramethylbenzidine substrate (Boster, Wuhan, Hubei Province, China) was added to the wells, which were left to stand for 20 minutes at room temperature. Absorbance values were read at 450 nm with 690 nm correction using a microplate reader (Multiskan Spectrum, Thermo Labsystems, Vantaa, Finland).
Extracellular signal-regulated kinase 1/2, phosphorylated extracellular signal-regulated kinase 1/2, and growth-associated protein 43 expression in Schwann cells as determined by western blot assay
Schwann cells (5 × 104/mL, 3 mL per well) were seeded into six-well plates until about 60% confluent. FK506 or rapamycin (300 μL) was added at final concentrations ranging from 1.53 nmol/L to 100 μmol/L. The control group was added to 300 μL of DMEM supplemented with 10% dimethyl sulfoxide. After 48 hours of incubation at 37°C under 5% CO2, the treated Schwann cells were collected, and subjected to western blotting to analyze specific proteins in the cells. All protein samples from the cells were denatured, loaded onto sodium dodecyl sulfate-polyacrylamide gradient (4–20%) gels (Bio-Rad, Hercules, CA, USA), resolved by electrophoresis, and electroblotted onto membranes. The blots were incubated with rabbit anti-extracellular signal-regulated kinase 1/2, phosphorylated extracellular signal-regulated kinase 1/2, and growth-associated protein 43 IgG (R&D Systems) diluted 1:2,000 in 1% bovine serum albumin-PBS for 2 hours at room temperature. Subsequently, blots were incubated with anti-rabbit secondary antibody labeled with alkaline horseradish peroxidase-conjugated goat-anti-rabbit IgG (R&D Systems) diluted 1:3,000 in 1% bovine serum albumin-PBS for 45 minutes at room temperature. Specific protein bands on the blots were detected via horseradish peroxidase/H2O2-catalyzed luminol oxidation under alkaline conditions using an enhanced chemiluminescence system (Beyotime Institute of Biotechnology, Jiangsu Province, China) and then by autoradiography. The autoradiograms were scanned on an Epson Perfection V300 Photo Scanner (Epson, Nagano-ken, Japan) using Adobe Photoshop (Adobe Systems, Seattle, WA, USA). Absorbance of each band was determined using Alpha Image software (Alpha Innotech, San Leandro, CA, USA).
Statistical analysis
Data were analyzed with SPSS 15.0 software (SPSS, Chicago, IL, USA) and expressed as mean ± SEM of separate experiments (n > 3). The results were compared by one-way analysis of variance and Fisher's post hoc test. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This work was supported by the Major State Basic Research Development Program of China (973 Program), No. 2011CB606205; the National Natural Science Foundation of China, No. 51172171 and 51103112; the Key Project of Chinese Ministry of Education, No. 313041; the Natural Science Foundation of Hubei Province, No. 2013CFB354; the Fundamental Research Funds for the Central Universities, No. WUT: 2013-IV-099.
Conflicts of interest: None declared.
Copyedited by Apricò K, Raye W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- [1].Sango K, Watabe K. Immortalized adult rodent Schwann cells as useful tools for the study of peripheral nerve regeneration. Rinsho Shinkeigaku. 2013;53(11):1117–1119. doi: 10.5692/clinicalneurol.53.1117. [DOI] [PubMed] [Google Scholar]
- [2].Guest J, Santamaria AJ, Benavides FD. Clinical translation of autologous Schwann cell transplantation for the treatment of spinal cord injury. Curr Opin Organ Transplant. 2013;18(6):682–689. doi: 10.1097/MOT.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Xu H, Holzwarth JM, Yan Y, et al. Conductive PPY/PDLLA conduit for peripheral nerve regeneration. Biomaterials. 2014;35(1):225–235. doi: 10.1016/j.biomaterials.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Radtke C, Kocsis JD, Reimers K, et al. Sural nerve defects after nerve biopsy or nerve transfer as a sensory regeneration model for peripheral nerve conduit implantation. Med Hypotheses. 2013;81(3):500–502. doi: 10.1016/j.mehy.2013.06.020. [DOI] [PubMed] [Google Scholar]
- [5].García Medrano B, Barrio Sanz P, Simón Pérez C, et al. Regeneration of critical injuries of the peripheral nerve with growth factors. Rev Esp Cir Ortop Traumatol. 2013;57(3):162–169. doi: 10.1016/j.recot.2013.03.007. [DOI] [PubMed] [Google Scholar]
- [6].Saheb-Al-Zamani M, Yan Y, Farber SJ, et al. Limited regeneration in long acellular nerve allografts is associated with increased Schwann cell senescence. Exp Neurol. 2013;247:165–177. doi: 10.1016/j.expneurol.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tung TH. Tacrolimus (FK506): Safety and Applications in Reconstructive Surgery. Hand (N Y) 2010;5(1):1–8. doi: 10.1007/s11552-009-9193-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Yousuf S, Atif F, Kesherwani V, et al. Neuroprotective effects of Tacrolimus (FK-506) and Cyclosporin (CsA) in oxidative injury. Brain Behav. 2011;1(2):87–94. doi: 10.1002/brb3.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Price RD, Yamaji T, Matsuoka N. FK506 potentiates NGF-induced neurite outgrowth via the Ras/Raf/MAP kinase pathway. Br J Pharmacol. 2003;140(5):825–829. doi: 10.1038/sj.bjp.0705522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brenner MJ, Mackinnon SE, Rickman SR, et al. FK506 and anti- CD40 ligand in peripheral nerve allotransplantation. Restor Neurol Neurosci. 2005;23(3-4):237–249. [PubMed] [Google Scholar]
- [11].Udina E, Ceballos D, Gold BG, et al. FK506 enhances reinnervation by regeneration and by collateral sprouting of peripheral nerve fibers. Exp Neurol. 2003;183(1):220–231. doi: 10.1016/s0014-4886(03)00173-0. [DOI] [PubMed] [Google Scholar]
- [12].Kaynar K, Ersoz S, Aliyazicioglu R, et al. Is there any way to protect from tacrolimus-induced renal and pancreas injury? Clin Transplant. 2012;26(5):722–728. doi: 10.1111/j.1399-0012.2012.01603.x. [DOI] [PubMed] [Google Scholar]
- [13].Loar RW, Patterson MC, O’Leary PW, et al. Posterior reversible encephalopathy syndrome and hemorrhage associated with tacrolimus in a pediatric heart transplantation recipient. Pediatr Transplant. 2013;17(2):E67–70. doi: 10.1111/petr.12039. [DOI] [PubMed] [Google Scholar]
- [14].Jones JW, Jr, Ustüner ET, Zdichavsky M, et al. Long-term survival of an extremity composite tissue allograft with FK506-mycophenolate mofetil therapy. Surgery. 1999;126(2):384–388. [PubMed] [Google Scholar]
- [15].Guethoff S, Meiser BM, Groetzner J, et al. Ten-year results of a randomized trial comparing tacrolimus versus cyclosporine a in combination with mycophenolate mofetil after heart transplantation. Transplantation. 2013;95(4):629–634. doi: 10.1097/TP.0b013e318277e378. [DOI] [PubMed] [Google Scholar]
- [16].Jones NF, Hebebrand D, Buttemeyer R, et al. Comparison of long-term immunosuppression for limb transplantation using cyclosporine, tacrolimus, and mycophenolate mofetil: implications for clinical composite tissue transplantation. Plast Reconstr Surg. 2001;107(3):777–784. doi: 10.1097/00006534-200103000-00019. [DOI] [PubMed] [Google Scholar]
- [17].Myckatyn TM, Mackinnon SE. A review of research endeavors to optimize peripheral nerve reconstruction. Neurol Res. 2004;26(2):124–138. doi: 10.1179/016164104225013743. [DOI] [PubMed] [Google Scholar]
- [18].Myckatyn TM, Ellis RA, Grand AG, et al. The effects of rapamycin in murine peripheral nerve isografts and allografts. Plast Reconstr Surg. 2002;109(7):2405–2417. doi: 10.1097/00006534-200206000-00035. [DOI] [PubMed] [Google Scholar]
- [19].Parker EM, Monopoli A, Ongini E, et al. Rapamycin, but not FK506 and GPI-1046, increases neurite outgrowth in PC12 cells by inhibiting cell cycle progression. Neuropharmacology. 2000;39(10):1913–1919. doi: 10.1016/s0028-3908(00)00028-9. [DOI] [PubMed] [Google Scholar]
- [20].Spiekerkoetter E, Tian X, Cai J, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123(8):3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Merkel S, Mogilevskaja N, Mengel M, et al. Side effects of sirolimus. Transplant Proc. 2006;38(3):714–715. doi: 10.1016/j.transproceed.2006.01.044. [DOI] [PubMed] [Google Scholar]
- [22].Rodriguez Camargo DC, Link NM, Dames SA. The FKBP-rapamycin binding domain of human TOR undergoes strong conformational changes in the presence of membrane mimetics with and without the regulator phosphatidic acid. Biochemistry. 2012;51(24):4909–4921. doi: 10.1021/bi3002133. [DOI] [PubMed] [Google Scholar]
- [23].Ciancio G, Sageshima J, Chen L, et al. Advantage of rapamycin over mycophenolate mofetil when used with tacrolimus for simultaneous pancreas kidney transplants: randomized, single-center trial at 10 years. Am J Transplant. 2012;12(12):3363–3376. doi: 10.1111/j.1600-6143.2012.04235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zammit NW, Tan BM, Walters SN, et al. Low-dose rapamycin unmasks the protective potential of targeting intragraft NF-κB for islet transplants. Cell Transplant. 2013;22(12):2355–2365. doi: 10.3727/096368912X658737. [DOI] [PubMed] [Google Scholar]
- [25].Ravikumar B, Vacher C, Berger Z, et al. Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet. 2004;36(6):585–595. doi: 10.1038/ng1362. [DOI] [PubMed] [Google Scholar]
- [26].Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer's disease. PLoS One. 2010;5(4):e9979. doi: 10.1371/journal.pone.0009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Malagelada C, Jin ZH, Jackson-Lewis V, et al. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson's disease. J Neurosci. 2010;30(3):1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rangaraju S, Verrier JD, Madorsky I, et al. Rapamycin activates autophagy and improves myelination in explant cultures from neuropathic mice. J Neurosci. 2010;30(34):11388–11397. doi: 10.1523/JNEUROSCI.1356-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lagoda G, Sezen SF, Burnett AL. FK506 and rapamycin neuroprotect erection and involve different immunophilins in a rat model of cavernous nerve injury. J Sex Med. 2009;6(7):1914–1923. doi: 10.1111/j.1743-6109.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- [30].Scheib J, Höke A. Advances in peripheral nerve regeneration. Nat Rev Neurol. 2013;9(12):668–676. doi: 10.1038/nrneurol.2013.227. [DOI] [PubMed] [Google Scholar]
- [31].Dey I, Midha N, Singh G, et al. Diabetic Schwann cells suffer from nerve growth factor and neurotrophin-3 underproduction and poor associability with axons. Glia. 2013;61(12):1990–1999. doi: 10.1002/glia.22570. [DOI] [PubMed] [Google Scholar]
- [32].Steiner JP, Connolly MA, Valentine HL, et al. Neurotrophic actions of nonimmunosuppressive analogues of immunosuppressive drugs FK506, rapamycin and cyclosporin A. Nat Med. 1997;3(4):421–428. doi: 10.1038/nm0497-421. [DOI] [PubMed] [Google Scholar]
- [33].Udina E, Rodríguez FJ, Verdú E, et al. FK506 enhances regeneration of axons across long peripheral nerve gaps repaired with collagen guides seeded with allogeneic Schwann cells. Glia. 2004;47(2):120–129. doi: 10.1002/glia.20025. [DOI] [PubMed] [Google Scholar]
- [34].Mendonça HR, Araújo SE, Gomes AL, et al. Expression of GAP-43 during development and after monocular enucleation in the rat superior colliculus. Neurosci Lett. 2010;477(1):23–27. doi: 10.1016/j.neulet.2010.04.027. [DOI] [PubMed] [Google Scholar]
- [35].Silva HT, Jr, Felipe CR, Garcia VD, et al. Planned randomized conversion from tacrolimus to sirolimus-based immunosuppressive regimen in de novo kidney transplant recipients. Am J Transplant. 2013;13(12):3155–3163. doi: 10.1111/ajt.12481. [DOI] [PubMed] [Google Scholar]
- [36].Gillen JR, Zhao Y, Harris DA, et al. Rapamycin blocks fibrocyte migration and attenuates bronchiolitis obliterans in a murine model. Ann Thorac Surg. 2013;95(5):1768–1775. doi: 10.1016/j.athoracsur.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lonic A, Powell JA, Kong Y, et al. Phosphorylation of serine 779 in fibroblast growth factor receptor 1 and 2 by protein kinase C(epsilon) regulates Ras/mitogen-activated protein kinase signaling and neuronal differentiation. J Biol Chem. 2013;288(21):14874–14885. doi: 10.1074/jbc.M112.421669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Grasselli G, Strata P. Structural plasticity of climbing fibers and the growth-associated protein GAP-43. Front Neural Circuits. 2013;7:25. doi: 10.3389/fncir.2013.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kaneda M, Nagashima M, Mawatari K, et al. Growth-associated protein43 (GAP43) is a biochemical marker for the whole period of fish optic nerve regeneration. Adv Exp Med Biol. 2010;664:97–104. doi: 10.1007/978-1-4419-1399-9_12. [DOI] [PubMed] [Google Scholar]
- [40].Onodera N, Kakehata A, Araki I. Differential expression of GAP-43 protein in the rostral brain neurons of early chick embryos. Tohoku J Exp Med. 2013;231(4):293–298. doi: 10.1620/tjem.231.293. [DOI] [PubMed] [Google Scholar]
- [41].Carreau A, Gueugnon J, Benavides J, et al. Comparative effects of FK-506, rapamycin and cyclosporin A, on the in vitro differentiation of dorsal root ganglia explants and septal cholinergic neurons. Neuropharmacology. 1997;36(11-12):1755–1762. doi: 10.1016/s0028-3908(97)00160-3. [DOI] [PubMed] [Google Scholar]
- [42].Zhou Y, Xiong Y, Yuan SY. Effect of tacrolimus on growth-associated protein-43 expression in the hippocampus of neonatal rats with hypoxic-ischemic brain damage. Zhongguo Dang Dai Er Ke Za Zhi. 2009;11(1):65–68. [PubMed] [Google Scholar]
- [43].King MA, Hands S, Hafiz F, et al. Rapamycin inhibits polyglutamine aggregation independently of autophagy by reducing protein synthesis. Mol Pharmacol. 2008;73(4):1052–1063. doi: 10.1124/mol.107.043398. [DOI] [PubMed] [Google Scholar]
- [44].Cao GF, Liu Y, Yang W, et al. Rapamycin sensitive mTOR activation mediates nerve growth factor (NGF) induced cell migration and pro-survival effects against hydrogen peroxide in retinal pigment epithelial cells. Biochem Biophys Res Commun. 2011;414(3):499–505. doi: 10.1016/j.bbrc.2011.09.094. [DOI] [PubMed] [Google Scholar]
- [45].Cao L, Zhu YL, Su Z, et al. Olfactory ensheathing cells promote migration of Schwann cells by secreted nerve growth factor. Glia. 2007;55(9):897–904. doi: 10.1002/glia.20511. [DOI] [PubMed] [Google Scholar]
- [46].De Simone R, Ambrosini E, Carnevale D, et al. NGF promotes microglial migration through the activation of its high affinity receptor: modulation by TGF-beta. J Neuroimmunol. 2007;190(1-2):53–60. doi: 10.1016/j.jneuroim.2007.07.020. [DOI] [PubMed] [Google Scholar]
- [47].Cornejo M, Nambi D, Walheim C, et al. Effect of NRG1, GDNF, EGF and NGF in the migration of a Schwann cell precursor line. Neurochem Res. 2010;35(10):1643–1651. doi: 10.1007/s11064-010-0225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yue XJ, Xu LB, Zhu MS, et al. Over-expression of nerve growth factor-β in human cholangiocarcinoma QBC939 cells promote tumor progression. PLoS One. 2013;8(4):e62024. doi: 10.1371/journal.pone.0062024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Haag D, Zipper P, Westrich V, et al. Nos2 inactivation promotes the development of medulloblastoma in Ptch1(+/-) mice by deregulation of Gap43-dependent granule cell precursor migration. PLoS Genet. 2012;8(3):e1002572. doi: 10.1371/journal.pgen.1002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Huang F, Liu Z, Liu H, et al. GM1 and NGF modulate Ca2+ homeostasis and GAP43 mRNA expression in cultured dorsal root ganglion neurons with excitotoxicity induced by glutamate. Nutr Neurosci. 2007;10(3-4):105–111. doi: 10.1080/10284150701406752. [DOI] [PubMed] [Google Scholar]
- [51].Verge VM, Tetzlaff W, Richardson PM, et al. Correlation between GAP43 and nerve growth factor receptors in rat sensory neurons. J Neurosci. 1990;10(3):926–934. doi: 10.1523/JNEUROSCI.10-03-00926.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Van Hooff CO, Holthuis JC, Oestreicher AB, et al. Nerve growth factor-induced changes in the intracellular localization of the protein kinase C substrate B-50 in pheochromocytoma PC12 cells. J Cell Biol. 1989;108(3):1115–1125. doi: 10.1083/jcb.108.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lai HC, Wu MJ, Chen PY, et al. Neurotrophic effect of citrus 5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone: promotion of neurite outgrowth via cAMP/PKA/CREB pathway in PC12 cells. PLoS One. 2011;6(11):e28280. doi: 10.1371/journal.pone.0028280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yin YX, Lin Q, Sun HM, et al. Cytotoxic effects of ZnO hierarchical architectures on RSC96 Schwann cells. Nanoscale Res Lett. 2012;7:439. doi: 10.1186/1556-276X-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mahmoudi M, Azadmanesh K, Shokrgozar MA, et al. Effect of nanoparticles on the cell life cycle. Chem Rev. 2011;111:3407–3432. doi: 10.1021/cr1003166. [DOI] [PubMed] [Google Scholar]
- [56].Entschladen F, Drell TL, 4th, Lang K, et al. Analysis methods of human cell migration. Exp Cell Res. 2005;307(2):418–426. doi: 10.1016/j.yexcr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- [57].Zantl R, Horn E. Chemotaxis of slow migrating mammalian cells analysed by video microscopy. Methods Mol Biol. 2011;769:191–203. doi: 10.1007/978-1-61779-207-6_13. [DOI] [PubMed] [Google Scholar]
- [58].Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- [59].Abbi S, Guan JL. Focal adhesion kinase: protein interactions and cellular functions. Histol Histopathol. 2002;17(4):1163–1171. doi: 10.14670/HH-17.1163. [DOI] [PubMed] [Google Scholar]


