Abstract
Spinal cord injury is a major cause of disability with devastating neurological outcomes and limited therapeutic opportunities, even though there are thousands of publications on spinal cord injury annually. There are two major types of spinal cord injury, transaction of the spinal cord and spinal cord contusion. Both can theoretically be treated, but there is no well documented treatment in human being. As for spinal cord contusion, we have developed an operation with fabulous result.
Keywords: spinal cord contusion, astrocyte, oligodendrocyte, macrophage, neurosurgery
What is spinal cord contusion
Spinal cord contusion (SCC) is an injury caused by crushing of the cord with part of its tissue spared, particularly the ventral nerve fibers connecting the spinal cord rostral and caudal to the injury remain physically intact (Beattie and Bresnaham, 2000). The severity of the spinal cord contusion is mainly judged by the physiological status of the remaining nerve fibers, whether the action potentials can pass through smoothly, hampered, or totally lost.
Basic pathology
Primary and secondary injuries?
At the time the spinal cord is contused there is crushed spinal cord tissue together with bleeding (the primary injury). A bunch of deleterious substances ooze out from the primary injury site resulting in the secondary injury, enclosed by an astrocytic wall. The front of the secondary injury is led by an ischemic zone, which causes tissue destruction and expansion of the secondary injury. This process goes on and on till it reaches its final stage with liquefied cavities and fibrous trabeculae (Beattie and Bresnaham, 2000).
Reactive astrogliosis and glial scar after spinal cord injury
Glial scar (also referred as gliosis) is a hallmark of the secondary spinal cord injury (SCI), which walls the lesion center off the rest of the cord (Fawcett and Asher, 1999). Characterized by reactive astrogliosis, the glial scar is constituted by densely populated reactive astrocytes, mingled with oligodendrocyte precursors, meningeal cells, microglia, macrophages, endothelial cells, fibroblasts, and the extracellular matrix among these cells (Barnabé-Heider et al., 2010; Göritz et al., 2011). Given the dominant roles of reactive astrogliosis in the formation and function of glial scar, we mainly discussed recent progress on the reactive astrogliosis after SCI.
Phenotype of reactive astrocyte
Reactive astrocytes are usually recognized by their hypertrophic morphology and strong glial fibrillary acidic protein (GFAP) immunoreactivity. Recent studies have revealed more phenotypic features of reactive astrocytes, in comparison with normal quiescent astrocytes (Ridet et al., 1997; Sofroniew, 2009). (1) Expression of neural progenitor markers, such as Nestin. This indicates that reactive astrocytes may be dedifferentiated into neural stem cells, as demonstrated by our previous study (Lang et al., 2004), as well as others’ (Robel et al., 2011). (2) Expression and secretion of cytokines, such as tumor necrosis factor alpha and beta (TNF-α and TNF-β), interleukin 1 and 6 (IL-1, 6). (3) Expression of neurotrophic factors and axon growth modulators, such as insulin like growth factor-1, leukaemia inhibitory factor, ciliary neurotrophic factor, chondroitin sulfate proteoglycan (CSPG), and keratan sulfate proteoglycan (KSPG). The diverse phenotype of reactive astrocytes may reflect the heterogeneity of astrocytic population. GFAP is a commonly used marker of astrocyte. It has been reported that there is quite frequent turnover between filamentous and soluble forms of GFAP, and only the filamentous form can be immunostained by antibody against GFAP (Walz, 2000). This is particularly important for quantitative studies.
Mechanism of astrocyte reactivation
Although not fully elucidated, mechanisms underlying the initiation, maintenance and migration of reactive astrocytes have been gradually uncovered. Among many candidate extracellular factors, transforming growth factor-β (TGF-β), IL-1 and interferon-γ have been demonstrated to trigger astrocyte re-activation (Sofroniew, 2009; Schachtrup et al., 2010). In the astrocytic cytoplasm, mTOR-STAT3 signaling pathway and BMP-mir-21 axis have been shown to be vital for the gene expression profile of reactive astrocytes (Codeluppi et al., 2009; Sahni et al., 2010). Interfering any of these signaling can significantly reduce the glial scar formation after SCI. Cytoskeleton proteins, such as GFAP and vimentin are required to maintain the hypertrophic morphology of reactive astrocytes (Menet et al., 2003). Extracellular matrix metalloproteinase 9 have been identified to facilitate the migration of reactive astrocytes (Hsu et al., 2008).
Roles of astrogliosis
Reactive astrocytes is a double-edged sword in SCI. A well documented bad side is that they express multiple axon growth inhibiting (CSPG, KSPG) and repelling factors (EphB, Semaphorin 3A), which may be a “chemical barrier” that prevents the regenerating axons to pass through the lesion site (Silver and Miller, 2004). In addition, recent studies showed that reactive astrocytes can suppress the generation of oligodendrocyte by releasing BMP, and inhibit remyelination by secreting endothelin-1 (Wang et al., 2011; Hammond et al., 2014). The bright side of astrogliosis is that it can limit the expansion of cavity, modulate inflammation and innate immune reaction, uptake extracellular glutamate, facilitate angiogenesis and rebuild of blood-spinal barrier, provide trophic support to neurons (Rolls et al., 2009). Genetically depleting reactive astrocyte impedes the restoration of blood-spinal barrier, increases the infiltration of neutrophil, increases neuronal death and demyelination, and therefore results in worse locomotion recovery (Rolls et al., 2009).
Oligodendrocytes
Studies from the 1990s have revealed that oligodendrocytes are quite susceptible to damage after SCI, and are lost in acute necrosis and acute/subacute apoptosis as a response to various triggers (Casha et al., 2001; Kim et al., 2003; Almad et al., 2011). Thus, demyelination of residual axons within the spinal cord white matter is an important contributor to the secondary pathophysiology (Rossignol et al., 2007). As the myelinating cells in the central nervous system (CNS), a single oligodendrocyte can form the myelin structure for many axons from different neurons. Thereafter, the death of one OL may lead to wallerian degeneration of many axons, which greatly expands the primary injury and contributes to functional deficits.
Some of the earlier studies examining demyelination after SCI in cats and rodents determined that demyelination was fairly prevalent during the first 2 weeks after injury (Gledhill et al., 1973; Blight, 1985). In human tissue, demyelinated axons have been detected to a variable degree along lesion borders from 1 to 22 years after injury (Guest et al., 2005). The lack of large-scale, chronic demyelination after SCI is due, at least in part, to spontaneous remyelination by oligodendrocytes and Schwann cells.
Axon remyelination in the adult CNS was first documented by Richard and Mary Bunge (Bunge et al., 1960; Bunge et al., 1961). Subsequent work has consistently detected limited axon remyelination after SCI, typically beginning around 2 weeks after injury (Gledhill et al., 1973). Intriguingly, when axons are remyelinated by oligodendrocytes, the myelin is thinner and the internodes are shorter compared to normal myelin (Griffiths and McCulloch, 1983), which is regarded as abnormal myelination or dysmyelination (McDonald and Belegu, 2006). Dysmyelination is worse than no myelination because it adds noise to the system, and bad information can be misleading or cause miscommunication.
Although remyelination after SCI has been acknowledged for many years, identifying the cells responsible for remyelinating axons was not clear-cut. Blakemore and Keirstead revealed that mature oligodendrocytes, which are post-mitotic, do not contribute to remyelination (Blakemore and Keirstead, 1999). Instead, remyelination is mediated by endogenous proliferating progenitor cells. These oligodendrocyte progenitors cells (commonly called OPCs or polydendrocytes) are present throughout the adult gray and white matter, and respond to demyelination by proliferating and migrating into the demyelinated zone (Blakemore and Keirstead, 1999; Watanabe et al., 2002; Richardson et al., 2011). Adult OPCs, typically identified by nerve/glial antigen 2 expression, are thought to be a heterogeneous cell population, only a portion of which function as OPC and is involved in the remyelination process (Nishiyama et al., 2009; Sellers et al., 2009; Trotter et al., 2010; Richardson et al., 2011). To enhance the differentiation of adult nerve/glial antigen 2-glia into mature oligodendrocytes, researchers have identified the effective function for several molecules, such as EGFR signaling, neurogenic/gliogenic transcription factor and growth factors (Ohori et al., 2006; Aguirre et al., 2007; Whittaker et al., 2012), which can enhance oligodendrocyte generation and axonal myelination after SCI. Besides, recent strategies have used transplantation of pluripotent/glial restricted cells to improve remyelination after SCI.
The highlights of a few relevant studies that focus on are the following: Lee et al. (2005) conducted a systematic analysis of transplantation of OPCs at 1 week postinjury in a rat spinal contusion model. They showed that OPCs migrated around the injury site and differentiated into mature oligodendrocytes (not astrocytes or neurons). The transplants also improved functional recovery and increased the number of retrograde-labeled neurons in the brainstem. Keirstead et al. (2005) differentiated human ESCs into a pure population of OPCs in vitro and transplanted the cells at 1 week or 10 months after SCI in rats. The grafted OPCs survived, integrated, migrated, and differentiated into oligodendrocytes at both time points after injury. More recent data from the same group show exciting results when OPC transplants were combined with growth factor and chondroitinase treatment, not only enhanced OL formation by transplant cells, but also increased growth of descending axons were observed (Sharp et al., 2010).
The effective cellular replacement experiments carried on animal models have approached their translation into clinical practice. In 2010, the United Stated Food and Drug Administration approved a phase I multi-center trial planned by Geron Corporation, which is designed to assess the safety and tolerability of GRNOPC1 (hESC-Derived OPC Therapies) in patients with American Spinal Injury Association (ASIA) Impairment Scale grade A subacute thoracic spinal cord injuries (Sypecka, 2011). This clinical trial represents an exciting advancement in SCI treatment, as well as highlights the importance of the need for continued research centered on the mechanisms integral to endogenous myelination and to maximize repair by endogenous or transplanted cells.
Microglia/macrophage polarization in spinal cord injury
The inflammatory response which is elicited by injury is one of the most important processes after SCI (Hawhtorne and Popovich, 2011). At the time the spinal cord is injured the axons are mechanically damaged, together with neurons and glial cells (oligodendrocytes, astrocytes and microglia) (Chan, 2008). Within hours to days after the initial injury, a complex cascade of secondary injury processes follows. Secondary injury processes are the major pathological alterations which include regional blood flow and vessel obstruction, electrolyte homeostasis perturbations, edema, free radical generation, excitotoxicity, inflammation, and Wallerian degeneration of the axons after SCI (Ankeny and Popovich, 2009; Chan, 2008). The secondary injury finally causes the development of a fluid-filled cavity surrounded by a glial scar which is believed to be an obstacle for axonal regeneration (Schwab et al., 2006; Popovich and Longbrake, 2008). Among these pathological processes, inflammation plays a crucial role in communicating and influencing other pathological processes which determine the recovery of SCI (Chan, 2008; Hawthorne and Popovich, 2011).
Disruption of the blood-spinal cord barrier after SC leads to the recruitment of extrinsic leukocytes from the systemic circulation, such as neutrophils, monocytes/macrophages, lymphocytes, and natural killer cells, as well as mobilization of intrinsic microglia to the site of injury (Chan, 2008; Hawthorne and Popovich, 2011). Neutrophils arrive first at injured site between 3 to 24 hours post-injury and then subside quickly. Monocytes/macrophages infiltrate later, 2 to 3 days post-injury, reaching its summit at 1 to 2 weeks after SCI (Donnelly and Popovich, 2008; Hawthorne and Popovich, 2011). The infiltrated lymphocytes arrive the injured site quite late. Indeed, the activated T and B lymphocytes increase significantly at around 4 weeks post-injury and persistent for a long time in the mouse (Donnelly and Popovich, 2008; Hawthorne and Popovich, 2011).
Microglia are innate cells with delicate branching processes that include 10–20% of cells in the CNS (Ransohoff and Cardona, 2010; Saijo and Glass, 2011; Aguzzi et al., 2013). They will transform to macrophages during inflammation. Different from other glial cells in CNS, which derive from neural stem cell, microglia derives from macrophages produced by primitive hematopoiesis in the yolk sac. The primitive macrophages migrate to and become residents in the developing neural tube, where they give rise to microglia (Ginhoux et al., 2010). Microglia and bone-marrow derived macrophages represent two genetically distinct myeloid populations. The number of microglia is maintained by local progenitors, whereas the BM derived monocytes contribute to the mature microglial pool in the CNS (Aguzzi et al., 2013). During SCI. microglia are the first myeloid cells to response. After activation, the microglia cannot be distinguished from the recruited macrophages by their characteristics in molecular surface markers and morphology. Therefore, they are referred as the microglia/macrophages subpopulation in injured spinal cord (David and Kroner, 2011). Among the inflammatory cells in injured site, microglia/macrophages are central players in the inflammatory response which determine the micro-environment and play beneficial or detrimental effects in axonal regeneration after SCI (Chan, 2008; David and Kroner, 2011; Sierra et al., 2013).
Plasticity and diversity have long been known to be hallmarkers of the macrophages. Macrophages, as well as microglia, display a spectrum of activation stages. The two extreme phenotypes of macrophages are defined as the “classically activated” pro-inflammatory (M1) or “alternatively activated” anti-inflammatory (M2) cells (Kigerl et al., 2009; Cao and He, 2013). In response to lipopolysaccharide (LPS) and the pro-inflammatory cytokine interferon-γ, microglia/macrophages undergo M1 polarization characterized by the expression of pro-inflammatory cytokines such as, IL-12, IL-1β, TNF-α and cytotoxic mediators (reactive oxygen and nitrogen species), as well as increase their phagocytic and antigen-presenting capacities. In contrast, activating macrophages in the presence of IL-4 or IL-13 undergo M2 polarization characterized by expression of anti-inflammatory cytokines such as TGF-β and IL-10, which contribute to the termination of inflammation (Lawrence and Natoli, 2011; Sica and Mantovani, 2012). In SCI, microglia/macrophages and other cell types up regulate inducible nitric oxide synthase (iNOS) and pro-inflammatory mediators such as TNF-α, IL-1β and IL-6 in the first few days after injury (David and Kroner, 2011). During first week after SCI, M1 (CD16/32 positive) and M2 (arginase-1) microglia/macrophages coexist at the lesion site and most of microglia/macrophages are M1 cells, with only a transient and small number showing M2 polarization in contused spinal cord. One of the classic M2 markers, arginase 1, is transiently upregulated and returns to normal levels by 7 days post-injury. However, the level of another M2 marker, CD206 (mannose receptor), is significantly higher at 14 day post-injury. However, only M1 microglia/macrophages persist until day 28 post-injury in mice (Kigerl et al., 2009). In contrast, the M1 markers CD16 and CD32 are upregulated at 7 day and reduce significantly at 14 and 28 day after injury (Kigerl et al., 2009). Other reports show that iNOS expression and iNOS+ microglia/macrophages were reported to peak at day 1 post-injury, whereas the expression of arginase-1 and arginase-1+ microglia/macrophages were days 4 to 7 post-injury in rat clip compression model (Ahn et al., 2012). Schwartz group has also proved that M1 cells reach their summit at day 1-3 and reduce gradually, whereas the number of M2 cells are highest at days 7–14 post-injury (Shechter et al., 2013a). This discrepancy in macrophage phenotypes in the pathogenesis of SCI remains to be further studied in both mouse and rat models (Shin et al., 2013).
A network of signaling molecules, transcription factors, epigenetic mechanisms, and post-transcrptional regulators underlines the different forms of polarized macrophages. TLR engagement leads to NF-κB (P50/P65 heterodimers) activation and production of inflammatory mediators associated with M1 macrophages. However, NF-κB activation Canonical IRF/STAT signaling pathways are activated by interferons and TLR signaling to polarize macrophages toward the M1 phenotype via STAT1 or by IL-4 or IL-13 to polarize to the M2 phenotype via STAT6 (Sica and Mantovani, 2012; Lawrence and Natoli, 2011). The IL-4 type I and type II receptors activate STAT6, which in turn activates transcription of genes typical of M2 polarization, e.g., mannose receptor (Mrc1), resistin-like α (Retnla, Fizz1), and chitinase 3-like 3 (Chi3l3, Ym1). IL-10 activates STAT3-mediated expression of genes (Il10, Tgfb1, Mrc1) associated with an M2-like phenotype. STAT-mediated activation of macrophages is regulated by members of the SOCS family. Interferon-γ, in concert with TLR stimulation, and IL-4 upregulates SOCS3 and SOCS1, which in turn inhibits the action of STAT3 and STAT1, respectively. The nuclear receptor PPARγ is a master regulator of lipid metabolism in macrophages and inhibits pro-inflammatory gene expression through several mechanisms, including transrepression of NF-κB (Lawrence and Natoli, 2011). The bZIP transcription factors, CREB (cAMP-responsive element-binding protein) and C/EBP (CCAAT/enhancer-binding protein-β), had recently been shown to be essential for M2-associated genes expression (Lawrence and Natoli, 2011). Our research also found that Aldose Reductase deficiency causes CREB upregulation leading microglia/microphages to M2 polarization after SCI (in submission). In addition, epigenetic changes of upregulation of the histo demethylase JMJD3 promotes expression of M2 genes and inhibits M1 genes. miR-155, targeting at the IL-13Rα1 subunit, decreases a set of M2 gene expression (Sica and Mantovani, 2012).
There is general consensus that M1 and M2 can reciprocally switch to each other based on the environment they are facing. A population of macrophages has been identified following experimental autoimmune encephalomyelitis (EAE) lesions, which express both M1 and M2 markers in one cell. These markers are induced by IL-4 in mouse EAE models, suggesting that phenotypic switching might occur in vivo in macrophages (Shin et al., 2013). Schwartz's group (2013a, b) has provided evidence showing that the M2 macrophages found at the lesion site following SCI are derived from monocytes that enter the CNS through the choroid plexus and then traffic along the CSF and the central canal. On the contrary, monocytes that polarize to M1 enter through the spinal cord leptomeninges adjacent to the site of injury. This suggests that macropahges are already educated to M1 or M2 polarized macrophages based on the route they infiltrate into injured spinal cord (Shechter et al., 2013a, b).
The M1 and M2 macrophages play a different role in axonal growth. In vitro evidence indicates that M1 macrophages can directly induce neuronal death. iNOS-expressing M1 macrophages correlate with tissue damage in SCI. M1 macrophages may also have a negative impact on axon regeneration as expression of CSPG, which is a potent inhibitor of axon growth and regeneration, is 17-fold higher in M1 macrophages than in M2 cells (David and Kroner, 2011; Kigerl et al., 2009). M2 macrophages are often described as anti-inflammatory cells as they express high level of IL-10 and TGF-β (David and Kroner, 2011). M2 macrophages seem to have no adverse effect on neurons and can promote growth of axons across inhibitory substrates that dominate sites of injury, probably by secreting neurotrophic factors (Hawthorne and Popovich, 2011).
SCI elicits an inflammatory response comprise of both resident and newly recruited circulating macrophages. The polarization of macrophages to M1 or M2 phenotypes determines the recovery of SCI. M2 macrophages are involved in the amelioration of CNS inflammation following SCI. The bias of M2 macrophages over M1 macrophages is a promising therapeutic method to promote recovery of SCI.
Treatment of spinal cord contusion
Based on the aforedescribed primary and secondary injuries, the logic solution is to debride the necrotic tissue enclosed in the astrocytic cavity at a relatively early stage, thereby virtually stops further expansion of the secondary injury. Meanwhiles the empty cavity will collapse and release the pressure on its surrounding issue, creating a favorable environment for functional recovery of the remaining tissue. Thus, we created an idea of “Early neurosurgery of spinal cord contusion” and put it into practice in collaboration with Kunming Army General Hospital, then the only hospital in China that had a department of spinal cord injury and could accommodate patients for rehabilitation for as long as 3 months. The ASIA grading system was adapted to classify the severity of cord injury. All ASIA-A (the most severe grade) patients with loss of all sensory and motor function below the spinal cord injury were non-selectively included, 30 cases in all. After routine MRI to determine the injury level, the injury of the spinal column was first treated, followed by bilateral laminectomy and internal fixation of the column. The spinal cord injured site was then detected, cut open, and debrided. The patients were kept in ICU for 3 days before transferring to the ward. After keeping the patients in bed for 17 days (17, arbitrary, routine practice of the Kunming Army General Hospital), a plan of rehabilitation was made for each patient according to his or her standing or walking ability, which will be revised in time according to the patient's improvement. The rehabilitation lasted for 3 months. Any medication that might affect functional recovery was avoided. Otherwise it would be difficult to attribute the therapeutic effect solely to the surgery. Since locomotion is a crucial criterion for recovery, we designed our own I to X locomotion grading system, the larger number the better recovery (Figure 1). The final outcome of the treatment turned out to be much better than we could ever have imagined (Figure 2) The least recovered was able to stand on two legs with body weight support, but when he started to walk, he had to shift his bodyweight to one leg before the other leg could be moved forward. Then the knee joint of the weight bearing leg needed help to prevent from bending down (grade IV). The optimal operation time window is 4–14 days after injury, during which 70% were able to walk with a pair of crutches or even without any support (Figure 3) (Zhu et al., 2008).
Figure 1.
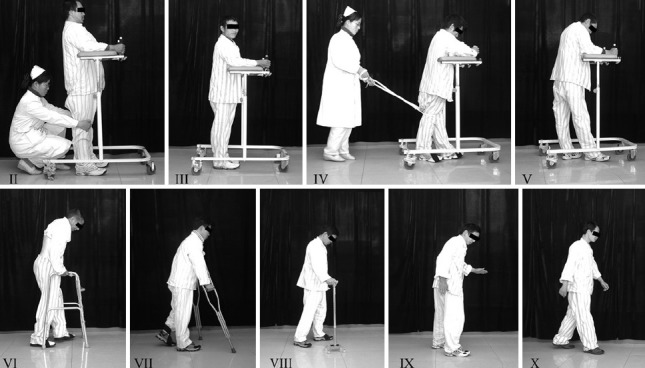
Our locomotion grading system.
The larger the Roman numeral the better the walking ability. Grade I refers to patients that cannot stand even with support, thus not shown in the figure.
Figure 2.
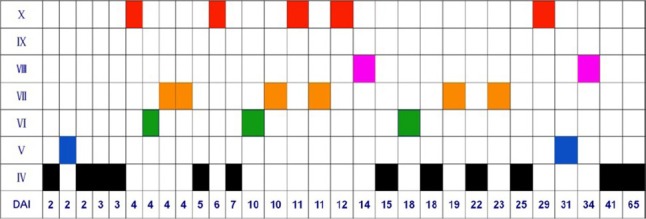
Summary of the results.
The roman numeral refer to the grade of the locomotion, DAI stands for days after injury before the patient was operated. By the end of the treatment, the patients had at least recovered to Grade IV (black boxes). The patient can stand firmly on a wheeled body support, but need support of the knee joint of the weight-bearing leg when walking. The best results occurred between DAI 4–14 days (the optimal operation time window), which included 13 cases, among which 9 cases (70%) can walk with a pair of crutches or even without any support.
Figure 3.
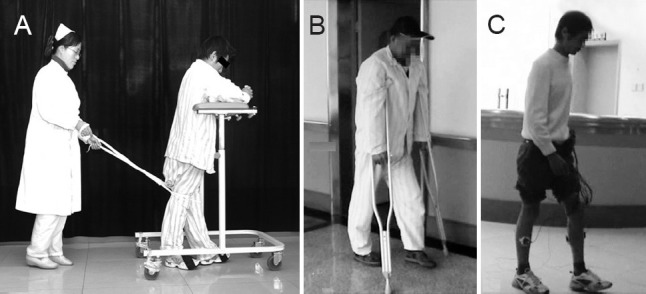
Examples of three grades IV, VII, and X of the patients.
(A) Grade IV, walking with help to fix the weight bearing leg. (B) Walking with a pair of cruches. (C) Free walking. The wires attached on his legs were for electromyelography.
Why is the operation not the earlier the better? The reason is that before 4 days after injury the necrotic cavity has not yet formed. After cutting open the injured spinal cord, what the surgeon can see is the mashed spinal cord tissue mixed with blood. The surgeon can only remove what he can see, but is not allowed to scoop the destroyed tissue beyond the field exposed, neither laterally nor up and down, because it may cause additional injury of the normal tissue. Thus, the débriment can never be thorough.
The best operation time window was defined by statistic analysis. But for individual patient the suitable day to receive operation should be specially tailored. How? We managed to figure out a way. After the injured level of the spinal cord is determined on the sagittal view of the MRI, condensed scanning of the injured cord follows immedately, once every few mm. Thus, the spinal cord injury can be clearly identified. If the necrotic cavity has not yet formed, repeat the scanning 5-7 days once, or twice.
Having proven the therapeutic value of the surgery, medication is no more a limitation. Use of helpful drugs is now encouraged. There are some that have often been used clinically. There is an ischemic area leading the front of the expending secondary injury. Our previous study found that there are two zones in the ischemic area. In the one adjacent to the injury most of the neurons have disappeared, while in the farther zone, although there are clear signs of ischemia, the morphology of the neurons appears basically normal (Figure 4) (Shen et al., 2009). It occurred to us that it might be possible to rescue the neurons in the second zone by enhancing blood circulation after the initial bleeding had stopped. Our studies have proved that this is the case by using betroxobin (Fan et al., 2013; Jia et al., 2010). This approach, therefore, can reasonably be a strategy in limiting the expansion of the secondary injury.
Figure 4.
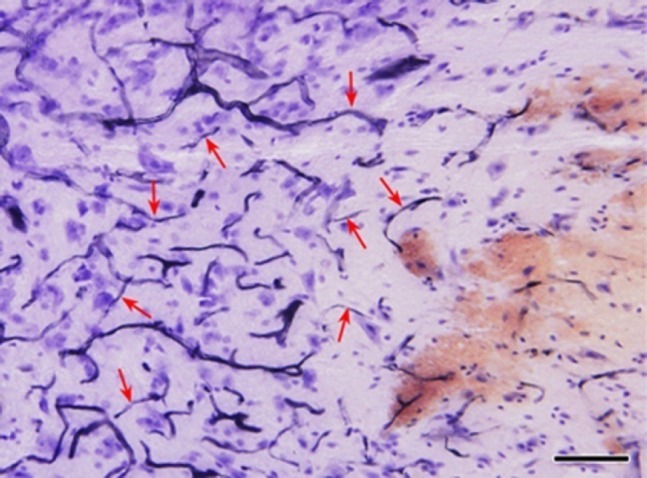
Sagittal section of spinal cord contusion (hematoxylin staining).
Red arrows, obstructed blood vessels. From right to left, reddish bleeding site of injury; pale ischemic zone with most of the neurons disappeared; an ischemic zone in which the morphology of most of the neurons remains largely normal. Bar = 100 μm.
We have also studied the effect of Lycium barbarum, a Chinese herbal drug, particularly renowned for centuries to be tonic to the eyes. Dr. Kwok-Fai So has, using modern scientific techniques, proved its beneficial effect in protecting retina neurons. We then studied its effect on spinal cord contusion in collaboration with Dr. Kwok-Fai So. We used the essential component of Lycium barbarum, the Lycium barbarum polysaccharide, in our study. We have demonstrated that that the major effect of LBP is to stimulate both the M1 and M2 types of the macrophages. The M1 type, which is deleterious, whereas the M2 type beneficial. In spinal cord injury M1 macrophage dominates for about 4 days, followed by dominance of the M2 type. When LBP was effective at the beginning of injury, the injury site examined at 7 day and 14 days were significantly larger than the vehicle control. Whereas administration of LBP started at 7 days after injury, when M2 type dominates, the lesion site was much smaller. We concluded that use of LBP could be very helpful if used after 7 days after injury (Zhang et al, 2013).
Footnotes
Conflicts of interest: None declared.
References
- 1.Aguzzi A, Barres BA, Bennett ML. Microglia: scapegoat saboteur or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aguirre A, Dupree JL, Mangin JM, Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat Neurosci. 2007;10:990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- 3.Ahn M, Yang W, Kim H, Jin JK, Moon C, Shin T. Immunohistochemical study of arginase-1 in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. Brain Res. 2012;1453:77–86. doi: 10.1016/j.brainres.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 4.Almad A, Sahinkaya FR, McTigue DM. Oligodendrocyte fate after spinal cord injury. Neurotherapeutics. 2011;8:262–273. doi: 10.1007/s13311-011-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ankeny DP, Popovich PG. Mechanisms and implications of adaptive immune responses after traumatic spinal cord injury. Neuroscience. 2009;158:1112–1121. doi: 10.1016/j.neuroscience.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnabé-Heider F, Göritz C, Sabelström H, Takebayashi H, Pfrieger FW, Meletis K, Frisén J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Beattie MS, Bresnahan IC. Cell death, repair, and recovery of function after spinal cord contusion injuries in rats. In: Kalb RC, Strittmatter SM, editors. In Neurobiology of Spinal Cord Injury. Humana Press; 2000. pp. 1–21. [Google Scholar]
- 8.Blakemore WF, Keirstead HS. The origin of remyelinating cells in the central nervous system. J Neuroimmunol. 1999;98:69–67. doi: 10.1016/s0165-5728(99)00083-1. [DOI] [PubMed] [Google Scholar]
- 9.Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2:299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- 10.Bunge MB, Bunge RP, Ris H. Ultrastructural study of remyelination in an experimental lesion in adult cat spinal cord. J Biophys Biochem Cytol. 1961;10:67–94. doi: 10.1083/jcb.10.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunge RP, Bunge MB, Rish H. Electron microscopic study of demyelination in an experimentally induced lesion in adult cat spinal cord. J Biophys Biochem Cytol. 1960;7:685–696. doi: 10.1083/jcb.7.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao L, He C. Polarization of macrophages and microglia in inflammatory demyelination. Neurosci Bull. 2013;29:189–198. doi: 10.1007/s12264-013-1324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience. 2001;103:203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- 14.Chan CC. Inflammation: beneficial or detrimental after spinal cord injury? Recent Pat CNS Drug Discov. 2008;3:189–199. doi: 10.2174/157488908786242434. [DOI] [PubMed] [Google Scholar]
- 15.Codeluppi S, Svensson CI, Hefferan MP, Valencia F, Silldorff MD, Oshiro M, Marsala M, Pasquale EB. The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci. 2009;29:1093–104. doi: 10.1523/JNEUROSCI.4103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–399. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 17.Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan H, Liu X, Tang H-B, Xiao P, Wang Y-Z, Ju G. Protective effects of Batroxobin on spinal cord injury in rats. Neurosci Bull. 2013;29:501–508. doi: 10.1007/s12264-013-1354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 20.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gledhill RF, Harrison BM, McDonald WI. Demyelination and remyelination after acute spinal cord compression. Exp Neurol. 1973;38:472–487. doi: 10.1016/0014-4886(73)90169-6. [DOI] [PubMed] [Google Scholar]
- 22.Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–242. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths IR, McCulloch MC. Nerve fibres in spinal cord impact injuries. Part 1. Changes in the myelin sheath during the initial 5 weeks. J Neurol Sci. 1983;58:335–349. doi: 10.1016/0022-510x(83)90093-x. [DOI] [PubMed] [Google Scholar]
- 24.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192:384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 25.Hammond TR, Gadea A, Dupree J, Kerninon C, Nait-Oumesmar B, Aguirre A, Gallo V. Astrocyte-derived endothelin-1 inhibits remyelination through notch activation. Neuron. 2014;81:588–602. doi: 10.1016/j.neuron.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hawthorne AL, Popovich PG. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics. 2011;8:252–261. doi: 10.1007/s13311-011-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu JY, Bourguignon LY, Adams CM, Peyrollier K, Zhang H, Fandel T, Cun CL, Werb Z, Noble-Haeusslein LJ. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci. 2008;28:13467–13477. doi: 10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia LY, Yao AH, Kuang F, Zhang YK, Shen XF, Ju G. Beneficial effect of the traditional Chinese Drug Shu-Xue-Tong on recovery of spinal cord injury in the rat. Evid Based Complement Alternat Med. 2010:862197. doi: 10.1155/2011/862197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K, Steward O. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim DH, Vaccaro AR, Henderson FC, Benzel EC. Molecular biology of cervical myelopathy and spinal cord injury: role of oligodendrocyte apoptosis. Spine J. 2003;3:510–519. doi: 10.1016/s1529-9430(03)00117-7. [DOI] [PubMed] [Google Scholar]
- 32.Lang B, Liu HL, Liu R, Feng GD, Jiao XY, Ju G. Astrocytes in injured adult rat spinal cord may acquire the potential of neural stem cells. Neuroscience. 2004;128:775–783. doi: 10.1016/j.neuroscience.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 33.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 34.Lee KH, Yoon DH, Park YG, Lee BH. Effects of glial transplantation on functional recovery following acute spinal cord injury. J Neurotrauma. 2005;22:575–589. doi: 10.1089/neu.2005.22.575. [DOI] [PubMed] [Google Scholar]
- 35.McDonald JW, Belegu V. Demyelination and remyelination after spinal cord injury. J Neurotrauma. 2006;23:345–359. doi: 10.1089/neu.2006.23.345. [DOI] [PubMed] [Google Scholar]
- 36.Menet V, Prieto M, Privat A, Giménez y, Ribotta M. Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci U S A. 2003;100:8999–9004. doi: 10.1073/pnas.1533187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishiyama A, Komitova M, Suzuki R, Zhu X. Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci. 2009;10:9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]
- 38.Ohori Y, Yamamoto S, Nagao M, Sugimori M, Yamamoto N, Nakamura K, Nakafuku M. Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J Neurosci. 2006;26:11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat Rev Neurosci. 2008;9:481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 40.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 41.Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70:661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 43.Robel S, Berninger B, Götz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- 44.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 45.Rossignol S, Schwab M, Schwartz M, Fehlings MG. Spinal cord injury: time to move? J Neurosci. 2007;27:11782–11792. doi: 10.1523/JNEUROSCI.3444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sahni V, Mukhopadhyay A, Tysseling V, Hebert A, Birch D, Mcguire TL, Stupp SI, Kessler JA. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J Neurosci. 2010;30:1839–1855. doi: 10.1523/JNEUROSCI.4459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saijo K, Glass T. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 48.Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwab JM, Brechtel K, Mueller CA, Failli V, Kaps HP, Tuli SK, Schluesener HJ. Experimental strategies to promote spinal cord regeneration--an integrative perspective. Prog Neurobiol. 2006;78:91–116. doi: 10.1016/j.pneurobio.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Sellers DL, Maris DO, Horner PJ. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci. 2009;29:6722–6733. doi: 10.1523/JNEUROSCI.4538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharp J, Frame J, Siegenthaler M, Nistor G, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cells. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013b;13:206–218. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]
- 53.Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity. 2013a;38:555–569. doi: 10.1016/j.immuni.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen XF, Zhao Y, Zhang YK, Jia LY, Ju G. A modified ferric tannate method for visualizing blood vessel and its usage in the study of spinal cord injury. Spinal Cord. 2009;47:852–856. doi: 10.1038/sc.2009.30. [DOI] [PubMed] [Google Scholar]
- 55.Shin T, Ahn M, Moon C, Kim S, Sim KB. Alternatively activated macrophages in spinal cord injury and remission: another mechanism for repair? Mol Neurobiol. 2013;47:1011–1019. doi: 10.1007/s12035-013-8398-6. [DOI] [PubMed] [Google Scholar]
- 56.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sierra A, Abiega O, Shahraz A, Neumann H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 59.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sypecka J. Searching for oligodendrocyte precursors for cell replacement therapies. Acta Neurobiol Exp (Wars) 2011;71:94–102. doi: 10.55782/ane-2011-1826. [DOI] [PubMed] [Google Scholar]
- 61.Trotter J, Karram K, Nishiyama A. NG2 cells: properties, progeny and origin. Brain Res Rev. 2010;63:72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walz W. Controversy surrounding the existence of discrete functional classes of astrocytes in adult gray matter. Glia. 2000;31:95–103. doi: 10.1002/1098-1136(200008)31:2<95::aid-glia10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Cheng X, He Q, Zheng Y, Kim DH, Whittemore SR, Cao QL. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31:6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watanabe M, Toyama Y, Nishiyama A. Differentiation of proliferated NG2-positive glial progenitor cells in a remyelinating lesion. J Neurosci Res. 2002;69:826–836. doi: 10.1002/jnr.10338. [DOI] [PubMed] [Google Scholar]
- 65.Whittaker MT, Zai LJ, Lee HJ, Pajoohesh-Ganji A, Wu J, Sharp A, Wyse R, Wrathall JR. GGF2 (Nrg1-beta3) treatment enhances NG2+ cell response and improves functional recovery after spinal cord injury. Glia. 2012;60:281–294. doi: 10.1002/glia.21262. [DOI] [PubMed] [Google Scholar]
- 66.Zhang YK, Wang J, Liu L, Chang R, C-C, So K-F, Ju G. The effect of Lycium barbarum on spinal cord injury, particularly its relationship with M1 and M2 macrophage in rats. BMC Complement Altern Med. 2013;13:67–92. doi: 10.1186/1472-6882-13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu H, Feng YP, Young W, You SW, Shen SF, Liu YS, Ju G. Early neurosurgical intervention of spinal cord contusion: an analysis of 30 cases. Chin Med J (Engl) 2008;121:2473–2478. [PubMed] [Google Scholar]


