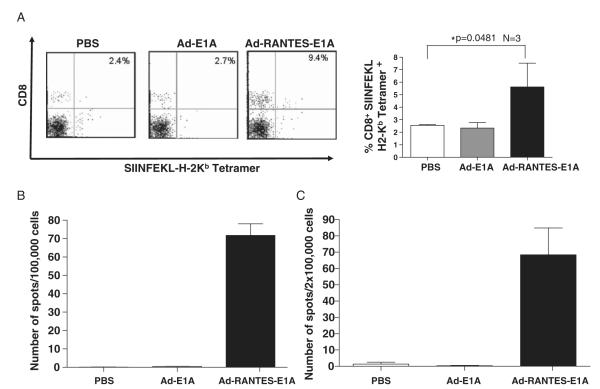
FIGURE 4.
Expansion of antigen-specific CTLs in Ad-RANTES-E1A–treated mice. A, Peripheral blood was collected from the E.G-7 mice 10 days after the final adenoviral immunization. Left panel, Peripheral blood lymphocytes were stained with CD8-FITC and SIINFEKL-H2-Kb tetramer. The percentage of double-positive cells is indicated in the quadrants. Right panel, Percentage of tetramer-positive CD8+ T cells in the peripheral blood of 3 mice in each group. *P<0.05 between tetramer-positive CD8 T cells in the blood of Ad-RANTES-E1A–treated group compared with Ad-E1A and PBS groups. B, Spleens were harvested from the JC-bearing mice 10 days after final immunization and frequency of IFN-γ secreting T cells in response to JC lysates was measured by EliSpot assay. Millipore MultiScreen-HA plates were coated with antimouse IFN-γ Ab. Splenocytes (105 cells/well) were cultured for 20 hours at 37°C in a 5% CO2 incubator in the complete medium with tumor lysates of JC. C, Total splenocytes from E.G-7–bearing mice were prepared 10 days after the last immunization and cocultured with 10 μg/ml of OT-I peptide for 20 hours. The frequency of SIINFEKL-specific T cells was determined by EliSpot assay. The results were evaluated with an automated EliSpot reader system. The number of IFN-γ–producing lymphocytes was evaluated in triplicate wells. Average ± SD for each mouse was calculated and the assays were repeated twice. Ab indicates antibody; CTL, cytotoxic T lymphocyte; EliSpot, enzyme-linked immunosorbent spot; FITC, fluorescein isothiocyanate; IFN, interferon; PBS, phosphate buffered saline; RANTES, regulated upon activation, normally T expressed, and presumably secreted.