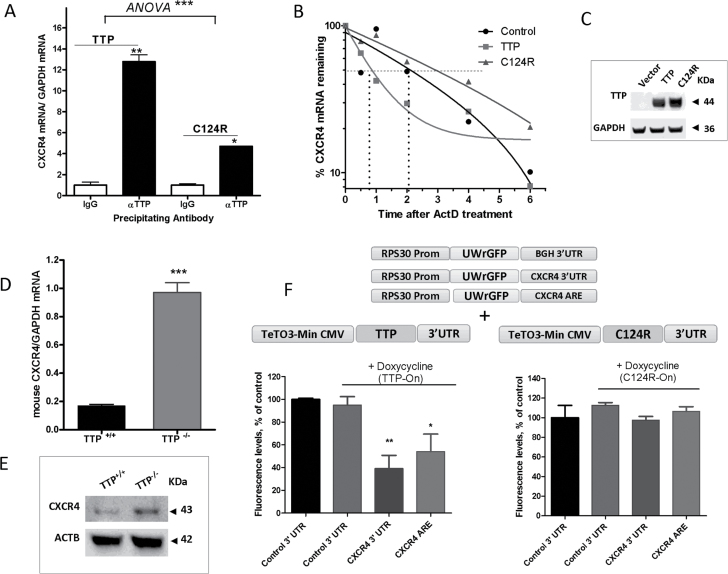
Fig. 2.
TTP regulation of CXCR4 mRNA and protein. (A) Quantitative PCR (qPCR) quantification of CXCR4 mRNA associated with TTP protein. MDA-MB-231 cells were transfected with TTP or C124R expression plasmids for 24h. Cells were lysed, and TTP and C124R proteins were immunoprecipitated using anti-TTP or normal IgG control antibody. Quantification of associated CXCR4 mRNA was performed by qPCR using a FAM-labeled human CXCR4 Taqman expression probe and normalized to a VIC-labeled GAPDH probe. Data are from one experiment representative of two independent experiments *P < 0.05, **P < 0.005 (Student’s t-test), ***P < 0.0001 [analysis of variance (ANOVA)]. (B) TTP regulation of CXCR4 mRNA half-life. MDA-MB-231 cells were transfected with TTP or C124R plasmid then treated the following day with actinomycin D (ActD, 5 μg/ml) for the indicated times then RNA was extracted for qPCR. Results are from one experiment representative of three independent experiments (C) Detection of overexpressed TTP and C124R proteins in MDA-MB-231 cells by western blotting using anti-TTP antibody. (D) qPCR quantification of CXCR4 mRNA in TTP+/+ and TTP− /− mouse embryonic fibroblasts (MEFs) using a FAM-labeled mouse CXCR4 probe and normalized to VIC-labeled mouse GAPDH, ***P < 0.0001 (Student’s t-test). (E) Representative western blot of CXCR4 protein in TTP+/+ and TTP− /− MEFs using anti-CXCR4 antibody. (F and G) TTP regulation of CXCR4 reporter expression. Tetracycline-inducible constructs were obtained by PCR of TTP and C124R plasmids, then 10ng of purified PCR product was transfected into HEK293 Tet-On Advanced cells and cotransfected with 25ng of RPS30 promoter-linked EGFP reporter plasmid constructs containing a control 3′-UTR, CXCR4 3′-UTR or CXCR4 ARE. Doxycycline (0.25 μg) was added 24h after transfection and GFP fluorescence was measured 24h after addition of doxycycline. Data were normalized to their corresponding non-doxycycline-induced samples (taken as 100%). Data are from one experiment representative of at least two independent experiments, * P < 0.05 and **P < 0.001 (Student’s t-test).