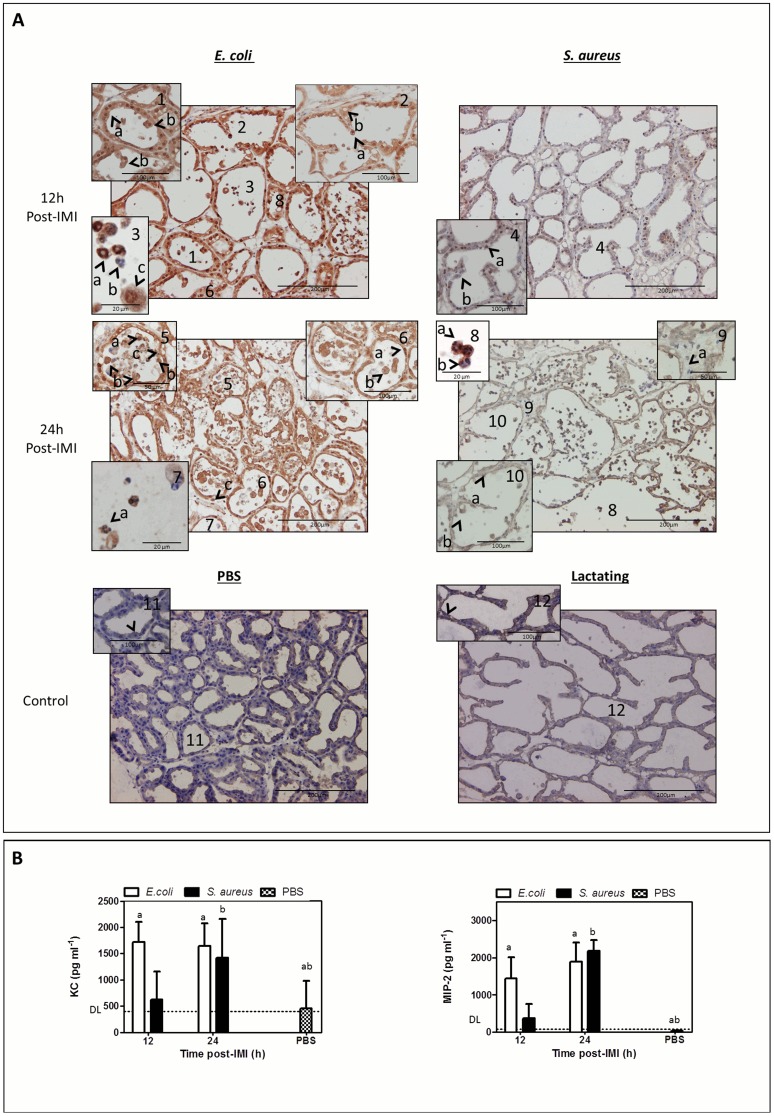
Figure 5. Differential nuclear translocation of mammary NF-kappaB p65, immune cell influx and damage at the mammary epithelium post-IMI with S. aureus compared to E. coli.
(A) At 12 h post-IMI, a nuclear translocation of NF-kappaB subunit p65 was observed in the epithelia of both E. coli (-1a-) and S. aureus-infected glands (-4a-). At this early time point, a strong cytoplasmatic NF-kappaB p65 signal was only seen post-IMI with E. coli. At 24 h post-IMI, NF-kappaB p65 was mainly detected in the cytoplasm in the mammary epithelium of both E. coli-(-6-) and S. aureus-infected glands (-10-). while at this time both sham-inoculated (PBS, -11-) and lactating (at 0 h,-12-) glands displayed very low basal levels of latent expressed cytoplasmic NF-kappaB p65. At 12 h post-IMI with E. coli the polymorphonuclear cells in the alveolar lumen could morphologically be identified as neutrophils (-3a- and -3b-), while these immune cells were still absent at that time post-IMI with S. aureus (-4-) or in sham-inoculated (PBS, -11-) and lactating (at 0 h,-12-) glands. In contrast, the influx of neutrophils could only be detected at 24 h post-IMI with S. aureus (-8-). A clear nuclear translocation of NF-kappaB p65 could be detected at 12 h post-IMI with E. coli and 24 h post-IMI with S. aureus (-3a- and -8a-) in these immune cells. At 24 h post-IMI with E. coli, epithelial damage at mammary alveoli was characterized by budding of mammary epithelia (-5b-) and resulted in shedded epithelial cells (-5a-) and empty spots in the luminal layer (-5c-), while at 24 h post-IMI with S. aureus only some cells started to protrude from the epithelial layer (-10b-). (B) IL-8 like chemokines (i.e. KC and MIP-2) confirmed this difference in kinetics of the neutrophil influx between both pathogens. Both chemokines were significantly induced in E. coli infected glands compared to sham-inoculated glands (PBS) already at 12 h post-IMI (a), while for S. aureus this strong induction was only seen at 24 h post-IMI (b). Letters of homogeneous subsets were marked if the difference between treatments were statistically significant (P<0.05)