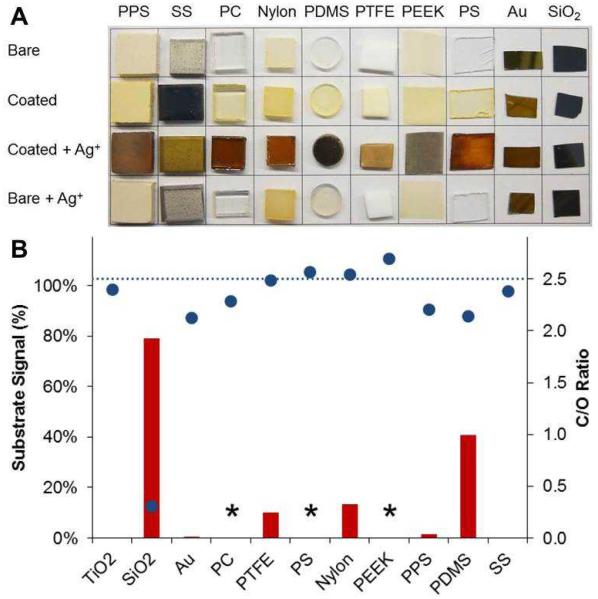
Fig. 2.
Spontaneous deposition of catechin-derived coatings on a variety of substrates. (A) Digital images of uncoated, coated, and AgNO3-treated substrates demonstrating versatility of catechin as a coating former on metal, ceramic, and polymer substrates. Darkening of substrates upon treatment with AgNO3 results from silver nanoparticle formation, confirming the presence and redox-active nature of the polyphenolic coating. (B) Surface chemical analysis of coated substrates by XPS. Inherent substrate signals are represented by red bars (100 % = no coating; * indicates the inability to differentiate coating from substrate by XPS), and carbon/oxygen (C/O) ratios are represented by blue symbols. Attenuation of substrate signal in conjunction with a C/O ratio similar to that of catechin (C/O = 2.5, blue dashed line) is indicative of successful coating formation.