Abstract
The study was conducted to investigate the role of vitamin E in the high altitude hypoxia-induced damage to the intestinal barrier in rats. Sprague-Dawley rats were divided into control (Control), high altitude hypoxia (HH), and high altitude hypoxia+vitamin E (250 mg/kg BW*d) (HV) groups. After the third day, the HH and HV groups were placed in a hypobaric chamber at a stimulated elevation of 7000 m for 5 days. The rats in the HV group were given vitamin E by gavage daily for 8 days. The other rats were given equal volume saline. The results showed that high altitude hypoxia caused the enlargement of heart, liver, lung and kidney, and intestinal villi damage. Supplementation with vitamin E significantly alleviated hypoxia-caused damage to the main organs including intestine, increased the serum superoxide dismutase (SOD) (p< 0.05), diamino oxidase (DAO) (p< 0.01) levels, and decreased the serum levels of interleukin-2 (IL-2) (p< 0.01), interleukin-4 (IL-4) (p<0.001), interferon-gamma (IFN-γ) (p<0.01) and malondialdehyde (MDA) (p<0.001), and decreased the serum erythropoietin (EPO) activity (p<0.05). Administration of vitamin E significantly increased the S-IgA (p<0.001) in ileum and significantly improved the expression levels of occludin and IκBα, and decreased the expression levels of hypoxia-inducible factor 1 alpha and 2 alpha (HIF-1α and HIF-2α), Toll-like receptors (TLR4), P-IκBα and nuclear factor-κB p65(NF-κB P65) in ileum compared to the HH group. This study suggested that vitamin E protectis from intestinal injury caused by high altitude hypoxia environment. These effects may be related to the HIF and TLR4/NF-κB signaling pathway.
Keywords: Hypoxia, Intestinal mucosa, Occludin, TLRs, Vitamin E
INTRODUCTION
High altitude hypoxia is a challenge for people residing in or visiting high altitudes. The exposure to high altitude hypoxia causes severe damage to different organs, especially the intestinal tract. There is increasing experimental evidence suggesting that hypoxia induced inflammation in the gastrointestinal tract [1,2,3]. Hypoxia may induce severe primary intestinal barrier dysfunction, promote bacterial and endotoxic translocation, and cause systemic inflammatory response; it is the major factor causing high-altitude multiple organ dysfunction syndromes [4]. The intestine is not only an important organ of digestion and nutrient absorption, but also has immunomodulatory, endocrine, and mucosal barrier functions. Intestinal mucosal barrier function is an important part of the barrier system of the body. The different structures, molecular mechanisms, and biological functions of each barrier allow them to collectively defense against the invasion of foreign antigens through combined signaling pathways [5]. Although high altitude hypoxia is the most common pattern, studies on effect of it on intestine are limited.
Vitamin E, a lipophilic vitamin, is an essential and complex nutrient for all species of animals. Vitamin E has many biological functions, the antioxidant function being the most important. As an antioxidant, vitamin E acts as a peroxyl radical scavenger, preventing the propagation of free radicals in tissue. Besides, it protects critical cellular structures against damage from oxygen free radicals and reactive products of lipid peroxidation [6,7]. In many species, T- and B-cell functions are impaired by vitamin E deficiency [8]. Vitamin E is absorbed from the gastrointestinal tract and enters the circulation via the lymph. Vitamin E deficiency may be associated with a discrete syndrome especially in intestinal fat malabsorption [9]. The exogenous provision of water and lipid-soluble antioxidant vitamins at the prescribed doses is an apparently safe and potentially effective intervention that can attenuate acute mountain sickness (AMS) and improve the physiological profile of mountaineers at high altitude [10]. Vitamin E supplementation results in preventing oxidative damage caused by high altitude stress [11]. There is growing evidence that free radicals mediated oxidative injury due to oxygen availability is an important factor in various pathologies at high altitude. Vitamin E is known to protect the cells from oxidative damage due to its potent antioxidant properties. Vitamin E recoveres the viability of vascular smooth mucle cells of rat aorta from gamma-radiaton injury [12]. However, the molecular mechanism of the protection effect of vitamin E on intestine mucosal barrier under high altitude hypoxia environment is still unclear.
The present study was conducted to investigate the role of vitamin E in the preservation of epithelial barrier function in the gastrointestinal tract of rat exposed high altitude hypoxia environment. We determined whether the small intestine was subjected oxidative damage during hypoxia accompanying changes in antioxidant status, evaluated changes in the expression of occludin,HIF-1α and HIF-2α in the ileum, and detected the serum related inflammatory mediators and erythropoietin (EPO) level. In addition, we investigated the role of TLR4/NF-κB signal pathway in the protective effect of vitamin E on intestinal barrier function damage induced by high altitude hypoxia.
METHODS
Instruments and reagents
The main instruments used in this study were hypobaric chamber (Guizhou Fenglei Aviation Ordnance Co., Ltd, China); fluorescence microscope (Nikon, Japan); ultraviolet spectrophotometer (HITACHI, Japan). The main regents used in this study were Vitamin E (Zhenjiang Medicine, China), MDA kit (Nanjing Jiancheng, China), SOD kit (Nanjing Jiancheng, China), Total protein kit (Nanjing Jiancheng, China), 4% formaldehyde (Beijing Dingguo Changsheng Biotechnology Co., Ltd.). Rat IL-2, IL-4, TNF-α, INF-γ, S-IgA, EPO and DAO ELISA kits (R&D systems, USA); TLR4 and NF-κB p65 rabbit polyclonal antibody, occludin rabbit polyclonal antibody, and β-actin mouse polyclonal antibody (Santa Cruz, USA); HIF-1α rabbit polyclonal antibody (EPI); HIF-2α rabbit polyclonal antibody (Abcam, USA); IκBα rabbit polyclonal antibody (Abcam, USA) and P-IκBα rabbit polyclonal antibody (CST, USA).
Experimental animals
This experiment was approved by the Institutional Animal Care and Use Committee of the Northwestern Polytechnical University (permit Number: 20130425) and was conducted in accordance with the National Institutes of Health guidelines for the care and use of experimental animals. Thirty adult male Sprague-Dawley rats (200±20 g) were purchased from the Experimental Animal Center of Xi'an Jiaotong University.
Experimental design
Adult male Sprague-Dawley (SD) rats were divided into control (Control), high altitude hypoxia (HH), and high altitude hypoxia+vitamin E group (HV). The experiment period was divided into two stages: on the first 3 days, all rats were placed in normal environment; then except for Control group, the other two groups were transferred into a hypobaric chamber at a simulated elevation of 7000 m for 5 days. During the 8 days, rats in group HG were given vitamin E (250 mg/kg B.W. d) by gavage daily. The rats in the control and HH groups received intragastric administration of an equal volume of saline. The chamber altitude was returned to sea level daily for 30 min to clean the cages and replenish the animal's food and water and give drugs. Besides, all the groups were free to food and water. All of the experimental animals had free access to food and water and were weighed daily.
Animal observation and sample collection
During the experiment, the food-intake, weight-gain, and their mental state, spontaneous activity were observed and recorded every day. Five days after exposure to high altitude hypoxia, the rats were anesthetized with ether and the abdomen was opened to collect 5 ml blood from abdominal aorta. The blood was centrifuged at 1,000 g for 10 min at 4℃, and the serum was separated and stored in Eppendorf tubes. The general condition of the rats and overall changes in the abdominal cavity were observed. Additionally, the heart, liver, spleen, lungs and kidney were removed from the rats and weighed. Approximately 5 cm of ileum were collected into RNAase-free tubes. All of the samples were frozen by immersion in liquid nitrogen and stored at -80℃ until needed for analysis.
Body, heart, liver, lung, kidney and spleen weight
The rats were sacrificed after completing the high altitude hypoxia. The body and organ weights of the heart, liver, lung, kidney and spleen were determined for each animal. The organ index was calculated as percentage of body weight.
Light microscopy for observation of intestinal morphology
Approximately 2 cm of duodenum, jejunum and ileum were obtained and cut open longitudinally and transversely. Then each collected intestinal segment was washed with normal saline immediately, fixed in 4% formaldehyde at 4℃ for 24 h, rinsed with phosphate buffered solution (PBS) and embedded in paraffin. The tissues were consecutively cut into 4-µm thick sections that were stained by hematoxylin and eosin (H&E). The intestinal morphology was observed using fluorescence microscope and the length and area of the intestinal villi were measured and calculated according to the following equation: Area=2πrh, where r represents the radius of the villus and h is the villus height.
Measurement of serum total SOD activity and MDA concentration
100 serum was used to detect the total SOD activity and MDA concentration. The SOD activity was measured using the hydroxylamine method. The serum MDA was measured with the thiobarbutiric acid (TBA) colorimetric method. The kits were used according to the manufacturer's instructions. The absorbance was measured at 550 nm and 532 nm respectively using an ultraviolet spectrophotometer. The activity of SOD was expressed as units per ml. The MDA content was calculated using the following formula: (nmol/ml)=[A (sample)-A (sample blank)]/[A (standard)-A (standard blank)], where A represents the absorbance value.
Detection of serum levels of IL-2, IL-4, EPO, IFN-γ, and DAO, and intestinal S-IgA levels
The levels of IL-2, IL-4, IFN-γ, EPO and DAO in the serum, and the S-IgA level in the intestine were detected using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacture's recommended protocol. A Synergy HT Multi-Detection Microplate Reader (Bio-Tek) was used to read the optical density at 450 nm. The concentrations of IL-2, IL-4, IFN-γ, EPO, S-IgA and DAO in the samples were determined using a standard curve.
Real-time RT-PCR for detection of occludin mRNA levels in ileum
Total RNA was extracted from the ileum with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The concentrations and purity of the RNA samples were assessed by ultraviolet spectrophotometer at 260 nm and 280 nm. The cDNAs were synthesized using a First-Strand cDNA Synthesis kit (Promega, Madison, WI, USA) according to the manufacturer's protocol. Real-time PCR was carried out using the Chromo4™ Real-time PCR System (Bio-Rad, USA) and SYBR® Premix Ex Taq™ kit (TAKARA). Each real-time PCR assay contained 2 µl cDNA template, 12.5 µl SRBR Premix Ex Taq, and 0.25 µl of each forward and reverse primer (10 µM) in a 25 µl reaction. The primers used in this study were as follows: occludin-F: 5'-GAGGCTTCAGAACGAGGCTATTT-3', occludin-R: 5'-CATGTCGGAGAGTAGAGGTTCGA-3'; β-actin-F: 5'-GGAGATTACTGCCCTGGCTCCTA-3', β-actin-R: 5'-GACTCATCGTACTCCTGCTTGCTG-3'. All runs were performed according to the PCR protocol (95℃ for 30 min, and 45 cycles of 95℃ for 5 s and 60℃ for 20 s). For each sample, real-time PCR was performed in triplicate, and the average threshold cycle (Ct) was calculated. A standard curve was generated to convert the Ct into copy number. Expression of occluding mRNA was normalized to actin mRNA expression. The average copy number of occludin mRNA was set to 1.0 in the control samples. The relative expression of occluding mRNA in the treated samples was determined as a fold increase compared with control samples.
Western blotting for detection of occludin, HIF-1α, HIF-2α, TLR4, IκBα, p-IκBα and NF-κB p65 expression
Ileum mucosal tissues (100 mg) were homogenized in 1 ml lysis buffer (Sangon Biotech, China). The total protein was extracted with a Protein Extract Kit (Beyotime, China) according to the manufacturer's instructions. The protein concentration was measured via a bicinchoninc acid assay using a BioRad protein microassay (BioRad, Hercules, CA, USA). An aliquot containing 30 µg of protein was diluted in loading buffer (loading buffer:sample=5:1, vole/vol) and heated to 98℃ for 10 min. The protein sample was separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The protein was transferred onto a 0.45 µm-pore polyvinylidene difluoride membrane (PVDF, Immuno-Blot, BioRad) at 100 V for 1 h. The membranes were blocked at room temperature for 2 h with 5% fat-free milk in PBS-T (PBS with 0.1% Tween-20). TLR4 (1:1000), NF-κB p65 (1:500), IκBα (1:1000), p-IκBα (1:1000), HIF-1α (1:1000), HIF-2α (1:1000), occludin (1:500) and β-actin (1:1000) primary antibodies were added, incubated at 4℃ overnight. After washing with PBS-T (0.1% BSA), the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary anti-rabbit antibody (diluted 1:10,000; Boster Co., Wuhan, China) for 2 h at room temperature. After additional washing, bound conjugates were detected with ECL Super-Signal™ West Pico substrate (Piece, Rockford, IL, USA). The proteins were visualized by exposing the blot to an X-ray film and were photographed with a digital camera. The net intensities of individual bands were measured using Quantity One (version 4.6.2). The relative expression levels of the proteins were expressed as the gray value of the target band over the gray value of β-actin in the same sample.
Statistical analysis
All data are shown as the means±S.E.M. The data were analyzed by a one-way analysis of variance (ANOVA) and by using Student's t-test (version 9.1, SAS, NC, USA). Differences were considered to be statistically significant at p<0.05.
RESULTS
General conditions including body and main organs
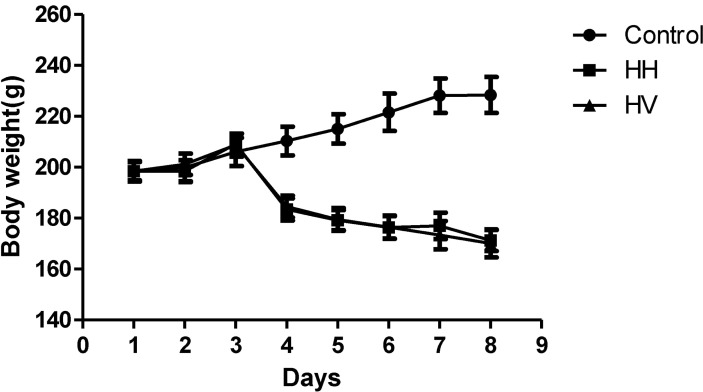
There were no animal deaths during the experiment. The rats in the control group were active, energetic and had no pathological reaction in the abdominal cavity that was visible to the naked eye. The rats in the HH and HV groups were less active and were notably more tired. Prominent swelling was observed in their intestinal canals, and the intestinal mucosa was congested. While in the hypobaric chamber, the food intake was significantly lower in the HH and HV groups than in the control group. Thus, reduced body weight was observed in all rats in the groups exposed to high altitude hypoxia (Fig. 1). Moreover, the eyes of the rats from the HH and HV groups were reddish brown. Table 1 showed that the heart, liver, kidney and lung index in group HH was significantly increased compared to the control group (p<0.05; p<0.01; p<0.01; p<0.001, respectively). However, the spleen index in the HH group decreased compared to the control group (p<0.05). Supplementation with vitamin E significantly alleviated high altitude hypoxia-induced liver and lung enlargement (p<0.05 and p<0.01, respectively) and spleen atrophy (p<0.05).
Fig. 1.
Effects of vitamin E and hypoxia on body weight in rats. Control, Control group; HH, High altitude hypoxia group; HV, High altitude hypoxia plus vitamin E group.
Table 1.
Effects of vitamin E and high altitude hypoxia on visceral index of rats (mg/g)
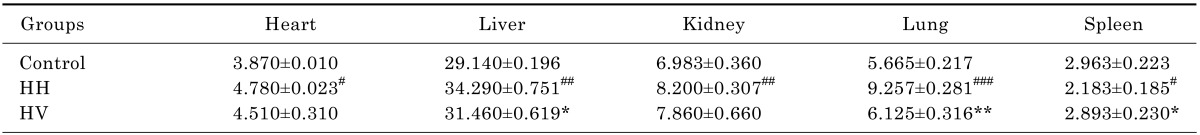
#p<0.05, ##p<0.01, ###p<0.001 vs Control; *p<0.05, **p<0.01 vs HH. Values are expressed as mean±S.E.M. (n=10). Control, Control group; HH, High altitude hypoxia group; HV, High altitude hypoxia plus vitamin E group.
Morphology of the intestinal tract
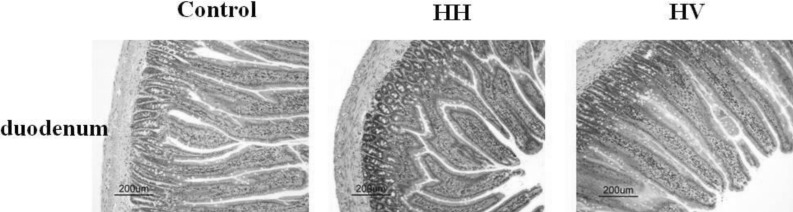
The intestinal villi with intact epithelia were dense and long and showed an ordered arrangement in the control group. Additionally, the intestinal mucosa was smooth and thick. There were no detected defects in the intestinal mucosa and villi using a light microscope. The intestinal villi in the HH group were sparse, short, and defective and had a scattered arrangement. In addition, the lodged and exfoliated villi became thinner than that in the control group (Fig. 2). The tight junctions between the intestinal epithelial cells were widened. The intestinal mucosa was exfoliated and showed signs of atrophy. Compared with the HH group, the intestinal villi in group HV were relatively intact and long. The intestinal mucosal also became thick. As shown in Table 2, the height of intestinal villi (p<0.001) and the thickness of mucosa (p<0.001) accompanying a decreased villi area (p<0.001) significantly decreased in the HH group compared to the control group. The height of intestinal villi (p<0.01), the thickness of mucosa (p<0.05), and villi area (p<0.01) significantly increased in group HV compared to the HH group.
Fig. 2.
Effect of vitamin E on intestinal morphology in rats from different groups. Control, Control group; HH, High altitude hypoxia group; HV, High altitude hypoxia plus vitamin E group (H&E, ×200).
Table 2.
Effect of vitamin E and high altitude hypoxia on intestinal villi and mucosa
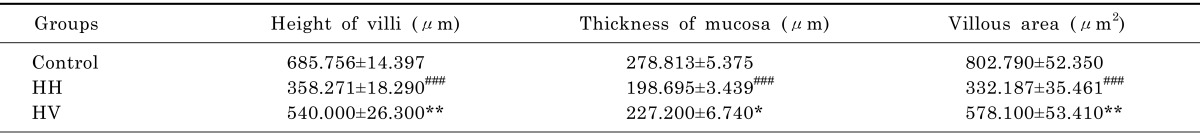
###p<0.001 vs Control; *p<0.05, **p<0.01, vs HH. Values are expressed as mean±S.E.M. (n=10). Control, Control group; HH, High altitude hypoxia group; HV, High altitude hypoxia plus vitamin E group.
Serum total SOD activity and MDA concentration
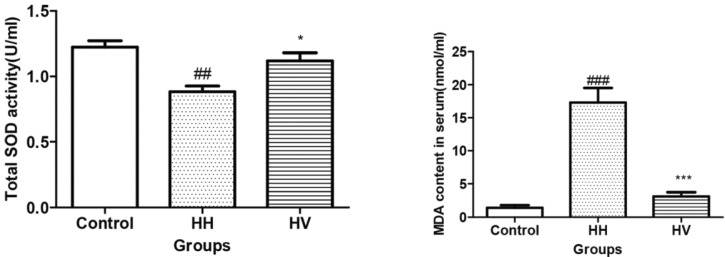
The serum total SOD activity in the HH group decreased dramatically compared to the control group (p<0.01) (Fig. 3). However, the serum total SOD activity in the HV group increased compared to the HH group (p<0.05). In contrast, the serum MDA content in the HH group was higher than the control group (p<0.001). However, the serum MDA content in HV group significantly decreased compared to the HH group (p<0.001).
Fig. 3.
Serum total SOD activity and MDA concentration. ##p<0.01, ###p<0.001 vs Control; *p<0.05, ***p<0.001 vs HH. Values are expressed as mean±S.E.M. (n=10). SOD, superoxide dismutase; MDA, malondialdehyde; Control, Control group; HH, High altitude hypoxia group; HV, High altitude hypoxia plus vitamin E group.
Serum IL-2, IL-4, IFN-γ, EPO and DAO levels, and intestinal S-IgA level
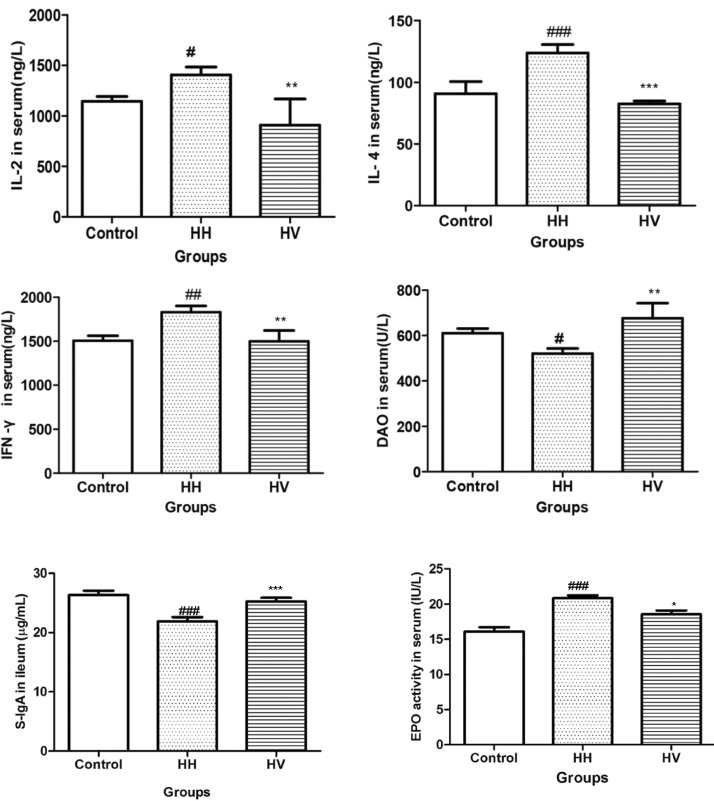
As shown in Fig. 4, the serum levels of IL-2 (p<0.05), IL-4 (p<0.001) and IFN-γ (p<0.01) in the HH group were significantly higher than those in the control group. This result suggests that hypoxia-induced inflammatory response. The serum levels of IL-2 (p<0.01), IL-4 (p<0.001) and IFN-γ (p<0.01) in the rats from the HV group were lower than those from the HH group. Moreover, the serum DAO levels (p<0.05) and intestinal S-IgA level (p<0.001) in the HH group decreased compared to the control group and increased significantly (DAO: p<0.01; S-IgA: p<0.001) in the HV group compared to the HH group. High altitude hypoxia exposure significantly increased the EPO activity (p<0.001) compared with the control group. Administration of Vitamin E significantly decreased the serum EPO activity (p<0.05) compared to the HH group.
Fig. 4.
Serum IL-2, IL-4, IFN-γ, EPO and DAO levels, and intestinal S-IgA levels. #p<0.05, ##p<0.01, ###p<0.001 vs Control; *p<0.05, **p<0.01, ***p<0.001 vs HH. Values are expressed as mean±S.E.M. (n=10). IL-2, interleukin-2; IL-4, interleukin-4; IFN-γ, interferon-gamma; DAO, diamine oxidase; EPO, erythropoietin; S-IgA, secretory Immunoglobulin A; Control, Control group; HH, High altitude hypoxia group; HV, High altitude hypoxia plus vitamin E group.
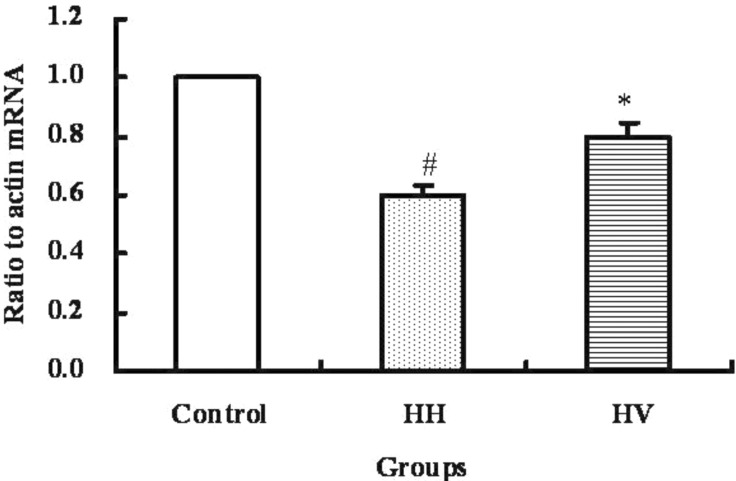
The mRNA level of occludin in ileum
The mRNA level of occludin in the ileum from each group was examined by real-time RT-PCR and the result was shown in Fig. 5. The occludin mRNA level in the ileum of the HH group was significantly decreased compared to the control group (p<0.05). The occludin mRNA level in the HV group was significantly higher than the HH group (p<0.05).
Fig. 5.
occludin mRNA levels in ileum. Values present means±S.E.M. (n=10). #p<0.05 vs. Control. *p<0.05 vs. HH. Control, Control group; HH, High altitude hypoxia group; HV, High altitude hypoxia plus vitamin E group.
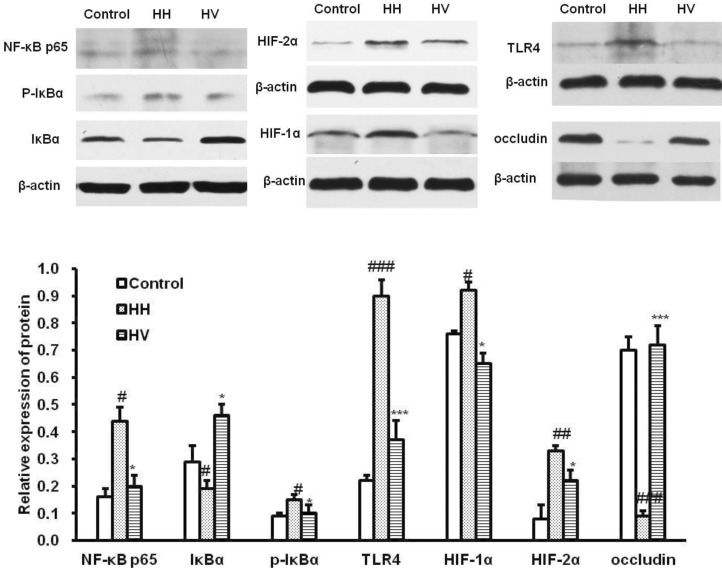
Occludin, HIF-1α, HIF-2α, TLR4,NF-κB p65, IκBα and p-IκBα protein expression in ileum
Western blot analysis (Fig. 6) showed that the expression level of NF-κB p65 (p<0.05), p-IκBα (p<0.05), HIF-1α (p<0.05), HIF-2α (p<0.01)and TLR4 (p<0.001) in the HH group were higher than those in the control group. Supplementation with vitamin E significantly decreased the expression levels of NF-κB p65 (p<0.05), p-IκBα (p<0.05), HIF-1α (p<0.05), HIF-2α (p<0.05) and TLR4 (p<0.001) compared with the HH group. However, the influence of high altitude hypoxia on occludin expression was contrary to it on above proteins. The expression level of occludin in group HH significantly decreased compared to the control group (p<0.001). Supplementation with vitaminm E rescued high altitude hypoxia-induced reduction of occludin, and the HV group showed increased occludin expression (p<0.001).
Fig. 6.
Occludin, HIFs, TLR4 and NF-κB p65 protein expression in ileum. #p<0.01, ##p<0.01, ###p<0.001 vs Control; *p<0.05, ***p<0.001 vs HH. Values are expressed as mean±S.E.M. (n=10). Values present means±S.E.M. (n=10). Control, Control group; HH, High altitude hypoxia group; HV, High altitude hypoxia plus vitamin E group.
DISCUSSION
Special geological and climatic environments might cause the increase of susceptibility to intestinal diseases for humans or animals exposed to high altitude. In the study, high altitude hypoxia caused the decrease of the body weight of rats, the enlargement of the heart, liver, kidney, and lung, and caused spleen atrophy. We further found that the intestinal mucosa and villi of rats was serious injured under high altitude hypoxia environment. This is consistent with previous reports of high altitude hypoxia-induced functional damage of the intestinal barrier [13]. Internal mucosa surfaces need to permit exchange of gas, fluid, and nutrients. Thus, as a trade-off, internal mucosal barrier are vulnerable and targeted by numerous pathogens that cause infection [14]. The mammalian intestinal tract harbors a highly microbial population that plays a major role in nutrition, metabolism, protection against pathogens, and development of the immune system [15,16]. Intact intestinal mucosa is so important that we have to seek some substance to protect intestine. Fortunately, the state of the intestinal mucosa and villi of rats exposed to high altitude hypoxia environment can be improved after administration of vitamin E (250 mg/kg B.W.·d), which may be related to the antioxidant activity of vitamin E. Vitamin E acts as a biological antioxidant at the cell membrane level. The ingestion of high doses of antioxidant nutrient such as vitamin E is considered a preventative measure against cell damage. Vitamin E has been reported to protect small intestinal crypt cell, mucosal height, and goblet cell from radiation [17].
High altitude is considered as an acute physiological stress leading to oxidative stress [10]. Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species (ROS) and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage [18]. Since high altitude hypoxia produced ROS directly, the counter balancing effect of the antioxidant enzymes is lost [19]. Oxidative damage and other related diseases occurred. SOD catalyzes the conversion of superoxide radical to hydrogen peroxide and protects body against the potential damage from superoxide radicals [20]. ROS also triggers lipid peroxidation. MDA is formed as an end product of lipid peroxidation and acts as an indicator of it [21,22]. The current results revealed that supplementation with vitamin E alleviated the increase of serum MDA concentration and the decrease of total SOD activity in serum caused by high altitude hypoxia. Indeed, antioxidant appeared to play a special role in protection of the intestinal cells from inflammation. It is believed that highly reactive free radicals literally disrupt cell membranes and cause inflammation in the gut. Vitamin E as one of the most abundant lipid-soluble antioxidants exists in two forms, as tocopherols and tocotrienols. α-tocopherol is the most potent antioxidant and plays an important role in peroxyl radical scavenging [8]. Vitamin E terminates the chain reaction of lipid peroxidation in membranes and lipoproteins. Therefore, vitamin E can alleviate the toxic effects of ROS, limits the effects of oxidant molecules on tissues and is active in the defense against oxidative cell injury by means of its being free radical scavengers [23].
The function of the intestinal barrier may be regulated by a network of multiple cytokines, including ILs, IFNs and TNF-α [24]. Inflammation is an important process in the pathogenesis of a variety of diseases [25]. An imbalance of pro-inflammatory cytokines and anti-inflammatory cytokines is another important mechanism of intestinal mucosal injury. High altitude hypoxia causes mucosal hyperpermeability in vivo. Subsequently, the activity of innate immune cells is increased, and this is associated with the activation of the mucosal immune system [26]. Along with other cytokines, like IL-4, IL-2 shares the common cytokine receptorγ chain, which is mutated in humans with X-linked severe combined immunodeficiency [27]. Increase of serum IL-2, IL-4 and INF-γ in rats exposed to high altitude hypoxia environment was observed in the study. INF-γ is a cytokine that is critical for innate and adaptive immunity against viral and bacterial infections [28,29]. However, administration of vitamin E inhibited the increase of IL-2, IL-4 and INF-γ. Tocopherol could affect the immune system through its antioxidant function either by decreasing reactive oxygen metabolites or by altering the formation of arachidonic acid metabolites, both of which have been shown to suppress immune responsiveness [30]. The mechanisms of vitamin E immunomodulation require further elucidation. Vitamin E has been influence cell function and homeostasis, and prostaglandin synthesis, which, through a number of interrelated processes mediate immune function [31].
DAO belongs to the class of copper-containing amine oxidases that convert primary amines to corresponding aldehydes, hydrogen peroxide, and ammonia. Human DAO may play an important role in histamine metabolism [32]. Among several tissues, DAO activity is particularly high in the upper portion of the small intestinal villi. Serum DAO level is employed as a useful marker of intestinal mucosal integrity to indicate the function and structure of the small intestine [33]. In the current study vitamin E treatment increased the DAO concentration of serum, which suggested that Vitamin E may play an important role in maintaining the integrity of the small intestine of rats exposed to high altitude hypoxia environment.
The intestinal tract is a complicated immune system. The S-IgA is the main component of mucosal secretions that can provide immune exclusion and defend mucosal surfaces from colonization and invasion by pathogens [34]. In the study, we observed the levels of S-IgA in intestine was increased in the vitamin E treatment group compared to the HH group. Tight junction (TJ) proteins including occludin, claudins, and cytoskeleton proteins play a critical role in maintaining the intestinal barrier integrity. Occludin is the first transmembrane protein discovered in the tight junction. Occludin plays a crucial role in the assembly or maintenance of epithelial tight junctions [35]. The C-terminal domain of occludin interacts with various intracellular proteins of tight junctions; this interaction is required for the assembly of occludin into the tight junctions [36]. The absence of occludin increases the ion permeability of TJs [37]. In the present study, high altitude hypoxia caused the significant decrease of occludin mRNA and protein expression. However, supplementation with vitamin E effectively alleviated the decrease of occludin expression levels in rats living in a high altitude hypoxia environment. These results indicated that administration of vitamin E can effectively alleviate high altitude hypoxia exposure leads to intestinal barrier dysfunction, decrease the intestinal permeability, and maintain the intestinal mucosal structure and barrier function integrity.
Exposure to high altitude hypoxia is characterized by a strong haematopoietic response, driven by the production of EPO, which increases oxygen carriage capacity. The physiological role of EPO is to adjust the pool of circulating erythrocytes to the actual oxygen demand. In the current study, high altitude exposure caused the increase of EPO in serum, which is consistent with previous results. Short or prolonged residency at high altitude is associated with increased secretion of EPO [38]. Increased EPO levels and the consequent augmented production of red blood cells is the best known systemic adaptation to reduced oxygen partial pressure (pO2). Intensive research during the last years revealed that the molecular mechanism behind the regulation of EPO is ubiquitous and has far more implications than first thought. EPO regulation results from the activation of the HIF-1 pathway under hypoxic conditions [39]. The marter regulator for many of the body's adaptive responses to hypoxia is HIF-1, a heterodimeric transcription factor comprising HIF-1α and HIF-1β. HIF-1α is both a mediator of the hypoxic response and a useful cellular marker of it. HIF-1α levels were elevated in the high altitude placenta [40]. HIF-1β is constitutively present in the nucleus, and when dimerized with HIF-1α is able to bind to hypoxia response elements (HREs) in the regulatory region of a number of genes, such as EPO [41]. Our results showed that the expression levels of HIF-1α and HIF-2α are significantly elevated in the ileum in response to high altitude hypoxia exposure when compared to the normal control group, which are consistent with the change of EPO. At high altitudes, hypoxia triggers HIF signaling [42,43]. These results further proved that the HIF signaling system imparts a dominant role in sensing O2 deprivation and subsequent gene expression, including the upregulation of EPO [44].
Toll-like receptors (TLRs) are a family of pattern-recognition receptors that play a key role in the innate immune system. As a key transmembrane protein closely related to bacterial recognition, TLR4 is thought to be involved in the first immune barrier of the gastrointestinal tract. NF-κB is the final effector molecule of the TLR4 signaling pathway. The increased expression of TLRs in the intestinal tissues during experimental necrotizing enterocolitis activated downstream NF-κB pathway and cytokine production [45]. NF-κB exists as a trimer of p65, p50, and a distinct lκB subunit. Activated NF-κB migrates to the nucleus for binding to DNA control elements and induces via mRNA the expression of genes encoding large number of cytokines [46]. Locally, these molecules can lead to apoptosis of intestinal mucosal epithelial cells and damage the tissues and organs of the intestinal tract. Our result suggested that high altitude hypoxia upregulated TLR4 expression and activated the TLR4/NF-κB signaling pathway. Moreover, the degree of activation of TLR4/NF-κB signaling pathway was consistent with changes in serum levels of IL-2, IL-4 and IFN-γ, and damage to the morphology and structure of the small intestine. Furthermore, we found that the expression of TLR4/NF-κB signaling proteins was inversely correlated with the expression level of occludin under hypobaric hypoxic conditions. This redistribution of occludin might damage barrier function. Thus, high altitude hypoxia-induced activation of TLR4/NF-κB may influence TJ complexes, eventually cause damage to the intestinal barrier. TLR4 and NF-κB expression increased in rat intestinal tissues after acute hypoxia exposure. Pyrrolidinedithiocarbamic acid (PDTC)-treatment reversed TLR4 and NF-κB upregulation and alleviated damage to the intestine tract and bacterial translocation [12]. These results suggest that TLR4/NF-κB signaling pathway may be related to the mechanism of damage to intestinal barrier function exposed to hypobaric hypoxia environment.
In summary, our results showed that a high altitude hypoxia environment causes pathological changes in the rat organs including damage to the intestinal villi, increased expression of cytokines and occludin, and activation of HIF and TLR4/NF-κB signaling pathway. Vitamin E can play a role to protect the intestinal barrier function, which may be associated with its antioxidant activity. The HIF and TLR4/NF-κB signal pathway may represent significant therapeutic targets for the prevention or treatment of intestinal barrier dysfunction and consequent intestinal diseases under high altitude hypoxia environment.
ACKNOWLEDGEMENTS
The study was supported by National Natural Science Foundation of China, No.31001012 and No.31101304, Programs for Agricultural Science and Technology Development of Shaanxi Province, China, No. 2013K02-16, Graduate Starting Seed Fund of Northwestern Polytechnical University (Z2014200) and Scientific Research Program Funded by Shaanxi Provincial Education Department (No.12JK0761). We are grateful to Wei Li, Chen Dong, Xiaobo Gao, Tiaotiao Han and Sheng Bao for their technical help.
ABBREVIATIONS
- SOD
superoxide dismutase
- DAO
diamino oxidase
- EPO
erythropoietin
- IL
interleukin
- IFN-γ
interferon-gamma
- MDA
malondialdehyde
- secretory IgA
S-IgA
- HIF
hypoxiainducible factor
- TLRs
Toll-like receptors
- NF-κB
nuclear factor-κB
- IκB
inhibitor of nuclear factor-κB
- TBA
thiobarbutiric acid
- ROS
reactive oxygen species
References
- 1.Zhou B, Yang DZ, Zhou QQ. The SEM observation of small intestinal mucosa in the rabbits under simulated high altitude hypoxia. Chin J Gastroenterol Hepatol. 2009;18:751–753. [Google Scholar]
- 2.Recavarren-Arce S, Ramirez-Ramos A, Gilman RH, Chinga-Alayo E, Watanabe-Yamamoto J, Rodriguez-Ulloa C, Miyagui J, Passaro DJ, Eza D. Severe gastritis in the Peruvian Andes. Histopathology. 2005;46:374–379. doi: 10.1111/j.1365-2559.2005.02102.x. [DOI] [PubMed] [Google Scholar]
- 3.Shen L. Functional morphology of the gastrointestinal tract. Curr Top Microbiol Immunol. 2009;337:1–35. doi: 10.1007/978-3-642-01846-6_1. [DOI] [PubMed] [Google Scholar]
- 4.Zhou QQ, Yang DZ, Luo YJ, Li SZ, Liu FY, Wang GS. Over-starvation aggravates intestinal injury and promotes bacterial and endotoxin translocation under high-altitude hypoxic environment. World J Gastroenterol. 2011;17:1584–1593. doi: 10.3748/wjg.v17.i12.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu WM, Zhang FX. Research advances in plateau hypoxia and gut barrier injury. World Chinese J Digestol. 2009;17:1432–1436. [Google Scholar]
- 6.Tang XL, Xu MJ, Li ZH, Pan Q, Fu JH. Effects of vitamin E on expressions of eight microRNAs in the liver of Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2013;34:1470–1475. doi: 10.1016/j.fsi.2013.03.353. [DOI] [PubMed] [Google Scholar]
- 7.Ernst IM, Pallauf K, Bendall JK, Paulsen L, Nikolai S, Huebbe P, Roeder T, Rimbach G. Vitamin E supplementation and lifespan in model organisms. Ageing Res Rev. 2013;12:365–375. doi: 10.1016/j.arr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Finno CJ, Valberg SJ. A comparative review of vitamin E and associated equine disorders. J Vet Intern Med. 2012;26:1251–1266. doi: 10.1111/j.1939-1676.2012.00994.x. [DOI] [PubMed] [Google Scholar]
- 9.Vavricka SR, Rogler G. Intestinal absorption and vitamin levels: is a new focus needed? Dig Dis. 2012;30(Suppl 3):73–80. doi: 10.1159/000342609. [DOI] [PubMed] [Google Scholar]
- 10.Bailey DM, Davies B. Acute mountain sickness; prophylactic benefits of antioxidant vitamin supplementation at high altitude. High Alt Med Biol. 2001;2:21–29. doi: 10.1089/152702901750067882. [DOI] [PubMed] [Google Scholar]
- 11.Ilavazhagan G, Bansal A, Prasad D, Thomas P, Sharma SK, Kain AK, Kumar D, Selvamurthy W. Effect of vitamin E supplementation on hypoxia-induced oxidative damage in male albino rats. Aviat Space Environ Med. 2001;72:899–903. [PubMed] [Google Scholar]
- 12.Lee JD, Choi HC, Kang YJ, Kim MS, Lee KY. Effects of Antioxidants on the gamma-radiation damage of the cultured vascular smooth mucle cells of rat aorta. Korean J Physiol Pharmacol. 2007;11:189–195. [Google Scholar]
- 13.Luo H, Guo P, Zhou Q. Role of TLR4/NF-κB in damage to intestinal mucosa barrier function and bacterial translocation in rats exposed to hypoxia. PLoS One. 2012;7:e46291. doi: 10.1371/journal.pone.0046291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013;34:155–161. doi: 10.1016/j.it.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Douglas-Escobar M, Elliott E, Neu J. Effect of intestinal microbial ecology on the developing brain. JAMA Pediatr. 2013;167:374–379. doi: 10.1001/jamapediatrics.2013.497. [DOI] [PubMed] [Google Scholar]
- 16.Rist VT, Weiss E, Eklund M, Mosenthin R. Impact of dietary protein on microbiota composition and activity in the gastrointestinal tract of piglets in relation to gut health: a review. Animal. 2013;7:1067–1078. doi: 10.1017/S1751731113000062. [DOI] [PubMed] [Google Scholar]
- 17.Roche M, Kemp FW, Agrawal A, Attanasio A, Neti PV, Howell RW, Ferraris RP. Marked changes in endogenous antioxidant expression precede vitamin A-, C-, and E-protectable, radiation-induced reductions in small intestinal nutrient transport. Free Radic Biol Med. 2011;50:55–65. doi: 10.1016/j.freeradbiomed.2010.10.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mena S, Ortega A, Estrela JM. Oxidative stress in environmental-induced carcinogenesis. Mutat Res. 2009;674:36–44. doi: 10.1016/j.mrgentox.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 19.Seth V, Banerjee BD, Chakravorty AK. Lipid peroxidation, free radical scavenging enzymes, and glutathione redox system in blood of rats exposed to propoxur. Pestic Biochem Physiol. 2001;71:133–139. [Google Scholar]
- 20.Uzun FG, Demir F, Kalender S, Bas H, Kalender Y. Protective effect of catechin and quercetin on chlorpyrifos-induced lung toxicity in male rats. Food Chem Toxicol. 2010;48:1714–1720. doi: 10.1016/j.fct.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 21.Najeed Q, Bhaskar N, Masood I, Wadhwa S, Kaur H, Ishaq S. Malondialdehyde (MDA) and superoxide dismutase (SOD) levels-distinguishing parameters between benign and malignant pleural effusions. Free Radic Antioxid. 2012;2:8–11. [Google Scholar]
- 22.Singh M, Thomas P, Shukla D, Tulsawani R, Saxena S, Bansal A. Effect of subchronic hypobaric hypoxia on oxidative stress in rat heart. Appl Biochem Biotechnol. 2013;169:2405–2419. doi: 10.1007/s12010-013-0141-2. [DOI] [PubMed] [Google Scholar]
- 23.El-Demerdash FM, Jebur AB, Nasr HM. Oxidative stress and biochemical perturbations induced by insecticides mixture in rat testes. J Environ Sci Health B. 2013;48:593–599. doi: 10.1080/03601234.2013.774998. [DOI] [PubMed] [Google Scholar]
- 24.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 25.Choi WJ, Kim SK, Park HK, Sohn UD, Kim W. Anti-Inflammatory and Anti-Superbacterial Properties of Sulforaphane from Shepherd's Purse. Korean J Physiol Pharmacol. 2014;18:33–39. doi: 10.4196/kjpp.2014.18.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu QJ, Zhou YM, Wu YN, Zhang LL, Wang T. The effects of natural and modified clinoptilolite on intestinal barrier function and immune response to LPS in broiler chickens. Vet Immunol Immunopathol. 2013;153:70–76. doi: 10.1016/j.vetimm.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Liao W, Lin JX, Leonard WJ. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol. 2011;23:598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 29.Hansen CH, Frøkiær H, Christensen AG, Bergström A, Licht TR, Hansen AK, Metzdorff SB. Dietary xylooligosaccharide downregulates IFN-γ and the low-grade inflammatory cytokine IL-1β systemically in mice. J Nutr. 2013;143:533–540. doi: 10.3945/jn.112.172361. [DOI] [PubMed] [Google Scholar]
- 30.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–1374. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 31.Muir WI, Husband AJ, Bryden WL. Dietary supplementation with vitamin E modulates avian intestinal immunity. Br J Nutr. 2002;87:579–585. doi: 10.1079/BJNBJN2002562. [DOI] [PubMed] [Google Scholar]
- 32.Elmore BO, Bollinger JA, Dooley DM. Human kidney diamine oxidase: heterologous expression, purification, and characterization. J Biol Inorg Chem. 2002;7:565–579. doi: 10.1007/s00775-001-0331-1. [DOI] [PubMed] [Google Scholar]
- 33.Hamada Y, Shinohara Y, Yano M, Yamamoto M, Yoshio M, Satake K, Toda A, Hirai M, Usami M. Effect of the menstrual cycle on serum diamine oxidase levels in healthy women. Clin Biochem. 2013;46:99–102. doi: 10.1016/j.clinbiochem.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Brandtzaeg P. Mucosal immunity: induction, dissemination, and effector functions. Scand J Immunol. 2009;70:505–515. doi: 10.1111/j.1365-3083.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- 35.Shang X, Wang P, Liu Y, Zhang Z, Xue Y. Mechanism of low-frequency ultrasound in opening blood-tumor barrier by tight junction. J Mol Neurosci. 2011;43:364–369. doi: 10.1007/s12031-010-9451-9. [DOI] [PubMed] [Google Scholar]
- 36.Rao R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann N Y Acad Sci. 2009;1165:62–68. doi: 10.1111/j.1749-6632.2009.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 38.Basu M, Malhotra AS, Pal K, Prasad R, Kumar R, Prasad BA, Sawhney RC. Erythropoietin levels in lowlanders and high-altitude natives at 3450 m. Aviat Space Environ Med. 2007;78:963–967. doi: 10.3357/asem.2085.2007. [DOI] [PubMed] [Google Scholar]
- 39.Höpfl G, Ogunshola O, Gassmann M. Hypoxia and high altitude. The molecular response. Adv Exp Med Biol. 2003;543:89–115. doi: 10.1007/978-1-4419-8997-0_7. [DOI] [PubMed] [Google Scholar]
- 40.Zamudio S, Wu Y, Ietta F, Rolfo A, Cross A, Wheeler T, Post M, Illsley NP, Caniggia I. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol. 2007;170:2171–2179. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedele AO, Whitelaw ML, Peet DJ. Regulation of gene expression by the hypoxia-inducible factors. Mol Interv. 2002;2:229–243. doi: 10.1124/mi.2.4.229. [DOI] [PubMed] [Google Scholar]
- 42.Chepelev NL, Willmore WG. Regulation of iron pathways in response to hypoxia. Free Radic Biol Med. 2011;50:645–666. doi: 10.1016/j.freeradbiomed.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 43.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, Rankin E, Vaulont S, Haase VH, Nizet V, Johnson RS. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Zhu L, Fatheree NY, Liu X, Pacheco SE, Tatevian N, Rhoads JM. Changes in intestinal Toll-like receptors and cytokines precede histological injury in a rat model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G442–G450. doi: 10.1152/ajpgi.00182.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber-Mzell D, Zaupa P, Petnehazy T, Kobayashi H, Schimpl G, Feierl G, Kotanko P, Hollwarth M. The role of nuclear factor-kappa B in bacterial translocation in cholestatic rats. Pediatr Surg Int. 2006;22:43–49. doi: 10.1007/s00383-005-1599-y. [DOI] [PubMed] [Google Scholar]