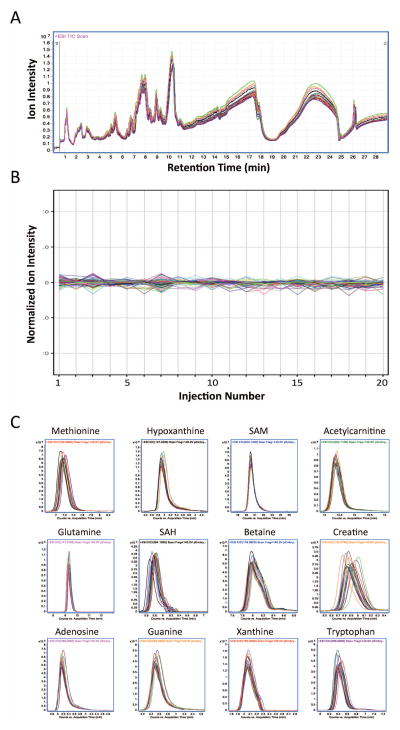
Figure 2. Technical reproducibility of whole-embryo metabolite profiling.
Panel A: Overlay of 20 repeated LC/MS analyses using Aqueous Normal Phase (ANP) chromatography and positive ionization MS for a quality control (QC) sample isolated from a pool of E9.5 mouse embryos. Panel B: Profile plot overlay of normalized ion intensity for 1,916 distinct metabolite features, quantified as a function of injection number for 20 repeated analyses. Panel C: Extracted ion chromatograms (EICs) peak overlay of some common metabolites observed in each of 20 repeated analyses, demonstrating reproducibility of relative ion abundances and retention times for these molecules. EIC overlays were generated using MassHunter Qualitative Analysis B.05.00; normalization and profile plot visualization were performed using MassProfiler Professional B12.0 (Agilent Technologies).