Abstract
AIM: To evaluate the effects of combined treatment of glutamine (Gln) and recombinant human growth hormone(rhGH) on intestinal barrier function following portal hypertension surgery.
METHODS: This study was designed as a prospective, randomized and controlled clinical trial. Forty two patients after portal hypertension surgery were randomly assigned into 2 groups: control group (n = 20) and supplemental group (adding Gln and rhGH, n = 22). Every patient received isocaloric and isonitrogenous standard total parenteral nutrition (TPN) starting 3 d after surgery for 7 d. Blood samples were obtained before surgery and at the 3rd and 10th day postoperatively. Host immunity was evaluated by measuring levels of CD4, CD8, CD4/CD8, IgG, IgM and IgA, and the inflammatory responses were determined by assessing IL-2, TNF-α and C-reactive protein (CRP) levels. Intestinal permeability and integrity was evaluated by L/M test and histological examination, respectively.
RESULTS: On postoperative d 10, CD4, CD4/CD8, IgG and IL-2 levels in supplemental group were significantly higher than those in control group (33.7 ± 5.5 vs 31.0± 5.4, P < 0.05, (1.17 ± 0.32 vs 1.05 ± 0.15, P < 0.05, 13.94 ± 1.09 vs 12.33 ± 1.33, P < 0.05, and 368.12± 59.25 vs 318.12 ± 45.65, P < 0.05, respectively), whereas the increase in serum TNF-α concentration was significantly reduced (41.02 ± 27.56 vs 160.09 ± 35.17, P < 0.05). The increase in L/M ratio was significantly lower in the supplemental group than in the control group (0.0166 ± 0.0017 vs 0.0339 ± 0.0028, P < 0.05). Moreover, mucosal integrity in the supplemental group was better than in the control group.
CONCLUSION: Postoperative administration of TPN supplemented with Gln and rhGH in patients after portal hypertension surgery improves immune function, modulates inflammatory response, prevents the intestinal mucous membrane from atrophy and preserves intestinal integrity.
Keywords: Liver cirrhosis, Portal hypertension, Total parenteral nutrition, Intestinal barrier function
INTRODUCTION
Surgical intervention and the use of intravenous alimentation over a long period of time will impair the barrier function of the intestine, increase mucosal permeability, decrease immunity, and lead to bacterial/endotoxin translocation[1,2]. If the intestinal barrier is impaired, the damage to the intestinal mucosa becomes more severe and bacterial translocation can lead to enterogenous infections, and even sepsis and multiple organ dysfunction syndrome (MODS) can ensue. These adverse affects are very common especially in patients with hepatic cirrhosis that underwent portal hypertension surgery. Glutamine (Gln) and recombinant human growth hormone(rhGH) protect the intestinal mucosa barrier after surgery. In this study, we determined the protective effect of Gln and rhGH on intestinal barrier function after portal hypertension surgery.
MATERIALS AND METHODS
Patients
This study was performed as a randomized, controlled trial. Forty-two patients with liver cirrhosis underwent portal hypertension surgery at the Third Affiliated Hospital of Sun Yat-sen University between June 1, 2004 and August 31, 2006. All patients gave their written consent to this study.
Diagnosis of cirrhosis was made according to the criteria revised in 1990 at the National Symposium on Cirrhosis in China. The following patients were excluded: hepatic function was Child-Pugh’s grade C, liver cancer, alcoholic cirrhosis or biliary cirrhosis, mental disease, severe cardiac, pulmonary, renal or cerebrovascular disease, diabetes, and those who developed severe complications.
Treatment
Patients were randomly divided into 2 groups: control group (n = 20) and supplemental group (n = 22). Patient’s demography from both groups is summarized in Table 1. Parenteral nutrition was initiated three days after surgery in both groups and lasted for seven days. Each patient received 125 kJ/kg body mass of calories every day of TPN solution. The ratio of glucose to lipid in this solution is 2:1, and nonprotein calorie to nitrogen (kcal/kg), 100:1. Multivitamins, electrolytes, trace elements and insulin were also included in the TPN solution. All nutrient solutions were prepared daily under aseptic conditions. Infusion was performed through a central venous catheter using an injecting micro pump. Gln (0.3 g/kg per day) was added into the TPN solution and rhGH (10 μ/d) was injected subcutaneously in the supplemental group.
Table 1.
Baseline characteristics of two groups
| Characteristics | Control group (n = 20) | Supplemental group (n = 22) |
| Male:Female | 15:5 | 17:5 |
| Age (yr) | 46.1 ± 2.1 | 44.3 ± 3.8 |
| Child-Pugh’s grade A | 11 | 10 |
| (No. of patients) | ||
| Child-Pugh’s grade B | 9 | 12 |
| (No. of patients) | ||
| Splenectomy | 13 | 15 |
| (No. of patients) | ||
| Splenectomy + EVL1 | 7 | 7 |
| (No. of patients) |
Endoscopic variceal ligation.
Monitoring of immunological and inflammatory responses
Immune function and inflammatory responses were determined before surgery and on d 3 and 10 after surgery. Three milliliters venous blood was taken for tests in the morning. Immune function was evaluated by measuring serum IgG, IgM, IgA and blood lymphocyte subsets (CD4, CD8 and CD4/CD8). Inflammatory responses were determined by assessing serum cytokine (IL-2, TNF-α, CRP) levels using radioimmunoassay kits (Sigma Inc., USA) according to the manufacturers’ recommendation. Lymphocyte subsets were assayed on a fluorescence activated cell scan flow cytometer (FACS calibur, Becton Dickinson, USA).
Lactulose/mannitol test
In the morning one day before surgery and on the 10th day postoperative, all patients received 10 g of lactulose and 5g mannitol dissolved in 50 mL water. Twenty-four hour urine was collected, the volume was recorded and 0.2 mL of mercury salicylosulfide added. Then 20 mL of the urine specimen was stored at -20°C until further tested. The lactulose and mannitol concentrations in the urine sample were measured by a high-performance liquid chromatograph (Waters Co, USA) using an ion-exchange column (Transgenomic Co, USA). The ratio between the two sugars was calculated.
Preparation of small intestine specimens
Duodenal mucosa biopsies were taken 2 d before and 10 d after surgery. Mucosa tissue was obtained in the descending part of duodenum and put into a 40 g/L neutral formaldehyde solution promptly for histological examination.
Histological examination of the intestinal mucosa
Specimens were embedded in paraffin, 4 μm sections were cut and stained with HE and analyzed with a HPIAS-1000 Multimedia Color Analysis System. Three low power (10 × 10) fields in each section were observed. The length of 5 villi and the depth of 5 crypts of the mucosa at 5 sites were analyzed. The average value was calculated and documented.
Expression of proliferating cell nuclear antigen (PCNA) index
Intestinal mucosa sections at a thickness of 4 μm were prepared and deparaffinized. Endogenous peroxidase was blocked with 3% H2O2 in methanol for 10 min, processed in boiling distilled water for 15 min, and then stained with PCNA staining kit (Zymed, South San Francisco, USA). Briefly, the sections were incubated with block solution and then with biotinylated mouse-peroxidase antibody. Streptavidin-peroxidase was used as a signal generator, and diaminobenzidine as a chromogen. The sections of specimens were counterstained with hematoxylin, dehydrated and mounted. A total of 10 high power fields (× 400) were counted for each sample. The cells with nuclei dyed into brown yellow were considered to be positive cells. The proportion of cells expressing PCNA in the nucleus was calculated as the number of labeled cells with respect to the total number of calculated enterocytes
Statistical analysis
The data is expressed as mean ± SD. Significance testing for univariate, between-group differences was performed using, where appropriate, the chi-square test, Fisher’s exact test, and Student’s t test. Statistical significance was defined as P < 0.05. Data analysis was performed using SPSS version 11.0.
RESULTS
Clinical
There were no differences in the baseline characteristics between the two groups as shown in Table 1. All subjects survived the duration of the study. TPN with or without combined Gln and rhGH treatments was well tolerated, with no short-term adverse effects identified on carbohydrate metabolism, or renal, hepatic, and cardiovascular status(data not shown).
Immune function
Serum IgG, IgM, and IgA concentrations and blood lymphocyte subset analyses are summarized in Table 2. Immune function was similar in both groups preoperatively and decreased postoperatively without any significant difference (P > 0.05). On d 10 postoperatively, however, IgG concentrations, CD4 expression, and CD4/CD8 ratio were significantly higher in the supplemental group than in the control group (P < 0.05).
Table 2.
Variations of immune and cytokine levels
|
Control group (n = 20) |
Supplemental group (n = 22) |
|||||
| d 0 | d 3 | d 10 | d 0 | d 3 | d 10 | |
| CD4 (%) | 30.2 ± 5.9 | 26.1 ± 3.2a | 31.0 ± 5.4b | 31.1 ± 3.2 | 27.6 ± 6.0a | 33.7 ± 5.5ce |
| CD8 (%) | 28.4 ± 1.5 | 31.4 ± 2.2a | 29.9 ± 1.5 | 27.8 ± 2.1 | 30.5 ± 2.4a | 28.8 ± 2.2 |
| CD4/CD8 | 1.05 ± 0.14 | 0.91 ± 0.22a | 1.05 ± 0.15b | 1.18 ± 0.14 | 0.92 ± 0.08a | 1.17 ± 0.32ce |
| IgG (g/L) | 13.34 ± 2.12 | 9.14 ± 1.36a | 12.33 ± 1.33b | 13.95 ± 1.68 | 10.12 ± 1.65a | 13.94 ± 1.09ce |
| IgA (g/L) | 2.55 ± 0.46 | 1.35 ± 0.21a | 1.98 ± 0.35b | 2.93 ± 0.74 | 1.33 ± 0.44a | 2.17 ± 0.31c |
| IgM (g/L) | 1.16 ± 0.35 | 0.75 ± 0.15a | 1.18 ± 0.20b | 1.18 ± 0.33 | 0.78 ± 0.24a | 1.28 ± 0.38c |
| IL-2 (pg/mL) | 256.21 ± 55.66 | 280.21 ± 48.32 | 318.12 ± 45.65 | 285.01 ± 71.78 | 290.21 ± 65.86 | 368.12 ± 59.25e |
| TNF-α (pg/mL) | 285.01 ± 42.25 | 385.98 ± 89.12 | 4 40.10 ± 56.52 | 280.10 ± 51.10 | 372.98 ± 85.22 | 321.12 ± 81.42e |
| CRP (mg/mL) | 6.4 ± 3.1 | 88.1 ± 25.6a | 42.2 ± 20.1b | 5.9 ± 5.2 | 65.1 ± 44.5a | 32.2 ± 15.2ce |
P < 0.05 vs d 0,
P < 0.05 vs d 3,
P < 0.05 vs control group.
Inflammatory responses
Circulating levels of IL-2, TNF-α and CRP are displayed in Table 2. Cytokine levels in both groups increased after surgery with no significant difference (P > 0.05). On postoperative d 10, levels of IL-2 were significantly higher in the supplemental group than in the control group (P < 0.05). However, the mean TNF-α and CRP concentrations were significantly lower in the supplemental group than in the control group (P < 0.05), and the increase in TNF-α levels postoperative was significantly lower in the supplemental group than in the control group (41.02 ± 27.56 vs 160.09 ± 35.17, P < 0.05).
Lactulose/mannitol (L/M) ratio
The L/M ratio increased in both groups after surgery (P < 0.05). However, the increase observed in the supplemental group was lower than in the control group (0.0166 ± 0.0017 vs 0.0339 ± 0.0028, P < 0.05) (Table 3).
Table 3.
Effect of Gln and rhGH on intestinal permeability (L/M ratio), morphology, and PCNA index before and after operation
|
Control group (n = 20) |
Supplemental group (n = 22) |
|||
| Preoperative | Postoperative | Preoperative | Postoperative | |
| L/M ratio | 0.0410 ± 0.0015 | 0.0749 ± 0.0051a | 0.0401 ± 0.0031 | 0.0567 ± 0.0033ac |
| Villus height (μm) | 352.4 ± 13.6 | 336.2 ± 11.6a | 359.7 ± 17.5 | 377.6 ± 14.6ac |
| Crypt depth (μm) | 115.0 ± 6.2 | 111.3 ± 3.9 | 117.7 ± 7.5 | 126.1 ± 7.2ac |
| PCNA (%) | 16.02 ± 2.06 | 15.65 ± 2.56 | 16.20 ± 1.87 | 24.08 ± 1.85ac |
P < 0.05 vs preoperative;
P < 0.05 vs control.
Morphology and morphometry of small intestinal mucous membrane
After seven days of TPN, the length of villi and depth of crypts in the supplemental group increased significantly compared with preoperative or control group (P < 0.05, respectively) (Table 3).
Expression of PCNA index of small intestinal mucous membrane
PCNA positive cells with a brown yellow-stained nucleus locate around the crypts. The PCNA index in the supplemental group increased significantly compared to preoperative or control group (P < 0.05) (Table 3, Figure 1).
Figure 1.
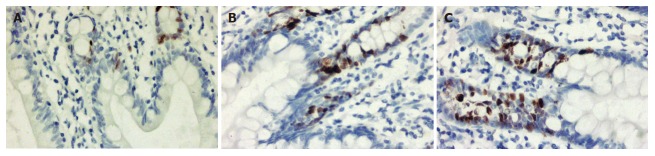
PCNA expression of intestinal mucosa. Brown nuclei are seen in PCNA positive cells. (DAB, × 400). A: Preoperative; B: Control, postoperative; C: Supplemental, postoperative.
DISCUSSION
The results of this trial show the benefits that Gln and rhGH supplemented TPN decreases intestinal permeability, causes hyperplasia of intestinal epithelial cells, and protects the morphology of small intestinal mucous membranes when compared to regular TPN following portal hypertension surgery.
Intestinal barrier malfunction is common in hepatic cirrhotic patients undergoing portal hypertension surgery. Surgical intervention usually results in impaired immune defense mechanisms and altered inflammatory responses[3,4]. The present study indicates that patients in both groups had lower immune function after surgery, and the markers descend further at d 3 postoperatively. With the increase in intestinal permeability, bacterial translocation and even MODS can ensue.
Increasing evidence suggests that nutritional support reduces postoperative complications and improves surgical outcomes. However, parenteral nutrition could meet the needs of other organs and tissues of the body, but not that of the intestinal mucosa. Seventy percent of the nutrients that intestinal epithelia need are absorbed directly from the intestinal lumen by mucosa cells. Qin and his colleagues[5] discovered that parenteral feeding in a 7-d period in experimental pancreatitis of dogs caused a significant damage of intestinal mucosa and bacterial translocation when compared to isonitrogenous and isocaloric enteral feeding. Similar results were reported by other scholars[6]. Newer strategies have focused on the use of specialized nutrition, including glutamine supplementation. Studies indicate that the addition of intravenous or enteral glutamine reduces infection rates[7-9] and length of hospital stay, and may improve mortality in intensive care unit (ICU) patients[10]. Glutamine has many biological functions. It comprises more than 50% of the body's free amino acid pool and is a precursor for synthesis of nucleic acids and glutathione. It is the main resource for rapid proliferating and dividing cells such as enterocytes, lymphocytes, and other immunocompetent cells etc. The structure and function of small intestinal mucosa is maintained and the increase in intestinal permeability is reduced when glutamine is supplemented to animals fed parenterally[11-13]. Our results confirm these findings. Additionally, glutamine could enhance body’s immunity through immune modulation. Results from a series of experiments and clinical investigations indicate that supplementation with glutamine parenterally and/or enterally has resulted in improved immune response when used in humans and animals[14,15].
Administration of growth hormone (GH) has been shown to have protein-anabolic effects in critically ill subjects following both elective and emergency surgery[16,17]. Moreover, GH can protect the intestinal mucosa barrier in obstructive jaundice which reduced intestinal translocation of bacteria and endotoxin[18,19]. Additionally, GH therapy has been shown to attenuate the skeletal muscle depletion of glutamine in critically ill patients[20,21], and it has been proposed that prevention of glutamine mobilization may contribute to loss of gut mucosal integrity, deteriorating sepsis, and multiple organ failure[22]. These findings perhaps indicate that GH treatments in the critically ill, highly catabolic patient should be combined with adequate provision of substrates, including amino acids and, in particular, glutamine.
In our study, the increase of L/M ratio after TPN was significantly lower in the experimental group when compared to the control group. The L/M test was used to measure the amount of excreted dual sugars in urine of 24 h. Because these two kinds of sugars are neither metabolized nor synthesized in the body, amounts of the two sugars being excreted from urine reflect the degree of intestinal permeability. The molecular weight of these two sugars is different. The test error can be reduced when using two sugars of different molecular weight instead of using only one for the test. If the ratio of the two sugars percentage in the tested group was significantly higher than that of normal, it could be concluded that the intestinal permeability in tested patients increased. Our results indicate that the combined use of Gln and rhGH prevents intestinal mucous membrane atrophy with preservation of intestinal integrity[23,24] following portal hypertension surgery. This finding possibly implies the synergistic action of an energetic substrate for the epithelium and a trophic hormone for the intestinal wall. However, the major limitation of the technique used in the present study is the morphology and morphometry of small intestinal mucous membrane. These measurements (an average of 5 slides/per specimen) are subjective and localized, and thus, errors may possibly exist. In fact, criticisms for the use of histological parameters are exactly due to subjectivism of the method. Therefore, all the calculation was done double-blinded by two experienced pathologists.
The mechanism of the increase in intestinal permeability is very complicated. It may be related to a number of inflammation mediators, such as cytokines[25], vasoactive amines[26], and oxygen free radicals[27,28]. In the present study, intestinal mucous membrane atrophy was found in every patient before operation. This may result from portal hypertension and malnutrition. However, it is uncertain if there is a correlation between the morphological alteration and the degree of increased intestinal permeability in our study. Further experiments should be performed.
Inflammation is essential for healing, immune processes and successful recovery after injury. However, uncontrolled systemic inflammatory responses lead to organ dysfunction and adverse outcome. Further, it has been suggested that injury alters the balance of protein synthesis by switching from constitutive to acute-phase proteins through the release of pro-inflammatory cytokines such as TNF-α and IL-6. This may be associated with a rapid decrease in nitrogen balance, with loss of lean body mass and with a catabolic response. In our study, we observed that combined use of Gln and rhGH increased serum immunoglobulin levels and increased the levels of CD4 cells and the CD4/CD8 ratio, which are disadvantageous to bacterial translocation and enterogenous infection. We also found that a Gln and rhGH supplemented nutritional support increased the serum level of IL-2 and decreased serum level of CRP and TNF-α. Our results suggest that Gln and rhGH can modulate postoperative immunosuppressive and inflammatory responses.
Although glutamine and recombinant human growth hormone with TPN could prevent the intestinal mucous membrane from atrophy with preservation of intestinal integrity, it is too early to conclude that they can prevent bacterial translocation. Nonetheless, despite the fact that none of our patients showed severe disorder in fluid and electrolytes during TPN, we still found TPN difficult for hepatic cirrhotic patients. Moreover, TPN is only suitable to support the ill subject, particularly in situations where enteral feeding is inadequate or impossible.
In conclusion, supplementation of parenteral nutrition with Gln and rhGH can protect the intestinal mucous membrane from atrophy, thus likely preserving intestinal integrity, and modulates postoperative immune- as well as inflammatory responses following portal hypertension surgery.
Footnotes
S- Editor Liu Y L- Editor Kremer M E- Editor Zhou T
References
- 1.Sun X, Spencer AU, Yang H, Haxhija EQ, Teitelbaum DH. Impact of caloric intake on parenteral nutrition-associated intestinal morphology and mucosal barrier function. JPEN J Parenter Enteral Nutr. 2006;30:474–479. doi: 10.1177/0148607106030006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang SC, Wang W, Ren WY, He BM, Zhou K, Zhu WN. Effect of cisapride on intestinal bacterial and endotoxin translocation in cirrhosis. World J Gastroenterol. 2003;9:534–538. doi: 10.3748/wjg.v9.i3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seehofer D, Rayes N, Schiller R, Stockmann M, Müller AR, Schirmeier A, Schaeper F, Tullius SG, Bengmark S, Neuhaus P. Probiotics partly reverse increased bacterial translocation after simultaneous liver resection and colonic anastomosis in rats. J Surg Res. 2004;117:262–271. doi: 10.1016/j.jss.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Rossi M, Sganga G, Mazzone M, Valenza V, Guarneri S, Portale G, Carbone L, Gatta L, Pioli C, Sanguinetti M, et al. Cardiopulmonary bypass in man: role of the intestine in a self-limiting inflammatory response with demonstrable bacterial translocation. Ann Thorac Surg. 2004;77:612–618. doi: 10.1016/S0003-4975(03)01520-0. [DOI] [PubMed] [Google Scholar]
- 5.Qin HL, Su ZD, Hu LG, Ding ZX, Lin QT. Effect of early intrajejunal nutrition on pancreatic pathological features and gut barrier function in dogs with acute pancreatitis. Clin Nutr. 2002;21:469–473. doi: 10.1054/clnu.2002.0574. [DOI] [PubMed] [Google Scholar]
- 6.Mosenthal AC, Xu D, Deitch EA. Elemental and intravenous total parenteral nutrition diet-induced gut barrier failure is intestinal site specific and can be prevented by feeding nonfermentable fiber. Crit Care Med. 2002;30:396–402. doi: 10.1097/00003246-200202000-00022. [DOI] [PubMed] [Google Scholar]
- 7.van den Berg A, van Elburg RM, Westerbeek EA, Twisk JW, Fetter WP. Glutamine-enriched enteral nutrition in very-low-birth-weight infants and effects on feeding tolerance and infectious morbidity: a randomized controlled trial. Am J Clin Nutr. 2005;81:1397–1404. doi: 10.1093/ajcn/81.6.1397. [DOI] [PubMed] [Google Scholar]
- 8.Déchelotte P, Hasselmann M, Cynober L, Allaouchiche B, Coëffier M, Hecketsweiler B, Merle V, Mazerolles M, Samba D, Guillou YM, et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med. 2006;34:598–604. doi: 10.1097/01.CCM.0000201004.30750.D1. [DOI] [PubMed] [Google Scholar]
- 9.Margaritis VG, Filos KS, Michalaki MA, Scopa CD, Spiliopoulou I, Nikolopoulou VN, Vagianos CE. Effect of oral glutamine administration on bacterial tanslocation, endotoxemia, liver and ileal morphology, and apoptosis in rats with obstructive jaundice. World J Surg. 2005;29:1329–1334. doi: 10.1007/s00268-005-7721-4. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths RD, Allen KD, Andrews FJ, Jones C. Infection, multiple organ failure, and survival in the intensive care unit: influence of glutamine-supplemented parenteral nutrition on acquired infection. Nutrition. 2002;18:546–552. doi: 10.1016/s0899-9007(02)00817-1. [DOI] [PubMed] [Google Scholar]
- 11.Khan J, Iiboshi Y, Cui L, Wasa M, Sando K, Takagi Y, Okada A. Alanyl-glutamine-supplemented parenteral nutrition increases luminal mucus gel and decreases permeability in the rat small intestine. JPEN J Parenter Enteral Nutr. 1999;23:24–31. doi: 10.1177/014860719902300124. [DOI] [PubMed] [Google Scholar]
- 12.Clark EC, Patel SD, Chadwick PR, Warhurst G, Curry A, Carlson GL. Glutamine deprivation facilitates tumour necrosis factor induced bacterial translocation in Caco-2 cells by depletion of enterocyte fuel substrate. Gut. 2003;52:224–230. doi: 10.1136/gut.52.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Hulst RR, van Kreel BK, von Meyenfeldt MF, Brummer RJ, Arends JW, Deutz NE, Soeters PB. Glutamine and the preservation of gut integrity. Lancet. 1993;341:1363–1365. doi: 10.1016/0140-6736(93)90939-e. [DOI] [PubMed] [Google Scholar]
- 14.Kudsk KA, Wu Y, Fukatsu K, Zarzaur BL, Johnson CD, Wang R, Hanna MK. Glutamine-enriched total parenteral nutrition maintains intestinal interleukin-4 and mucosal immunoglobulin A levels. JPEN J Parenter Enteral Nutr. 2000;24:270–274; discussion 270-24;. doi: 10.1177/0148607100024005270. [DOI] [PubMed] [Google Scholar]
- 15.Wessner B, Strasser EM, Manhart N, Roth E. Supply of R-alpha-lipoic acid and glutamine to casein-fed mice influences the number of B lymphocytes and tissue glutathione levels during endotoxemia. Wien Klin Wochenschr. 2006;118:100–107. doi: 10.1007/s00508-006-0568-3. [DOI] [PubMed] [Google Scholar]
- 16.Carroll PV, Jackson NC, Russell-Jones DL, Treacher DF, Sönksen PH, Umpleby AM. Combined growth hormone/insulin-like growth factor I in addition to glutamine-supplemented TPN results in net protein anabolism in critical illness. Am J Physiol Endocrinol Metab. 2004;286:E151–E157. doi: 10.1152/ajpendo.00122.2003. [DOI] [PubMed] [Google Scholar]
- 17.Sevette A, Smith RC, Aslani A, Kee AJ, Hansen R, Barratt SM, Baxter RC. Does growth hormone allow more efficient nitrogen sparing in postoperative patients requiring parenteral nutrition A double-blind, placebo-controlled randomised trial. Clin Nutr. 2005;24:943–955. doi: 10.1016/j.clnu.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Yang ZW, Li JG, Mao XG, Sun B, Tong ZS, Sun HY, Li XR, Cong YP. Effects of recombinant human growth hormone on intestinal translocation of bacteria and endotoxin in rats with obstructive jaundice. Hepatobiliary Pancreat Dis Int. 2005;4:445–449. [PubMed] [Google Scholar]
- 19.Lorenzo-Zúñiga V, Rodríguez-Ortigosa CM, Bartolí R, Martínez-Chantar ML, Martínez-Peralta L, Pardo A, Ojanguren I, Quiroga J, Planas R, Prieto J. Insulin-like growth factor I improves intestinal barrier function in cirrhotic rats. Gut. 2006;55:1306–1312. doi: 10.1136/gut.2005.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biolo G, Iscra F, Bosutti A, Toigo G, Ciocchi B, Geatti O, Gullo A, Guarnieri G. Growth hormone decreases muscle glutamine production and stimulates protein synthesis in hypercatabolic patients. Am J Physiol Endocrinol Metab. 2000;279:E323–E332. doi: 10.1152/ajpendo.2000.279.2.E323. [DOI] [PubMed] [Google Scholar]
- 21.Gamrin L, Essén P, Hultman E, McNurlan MA, Garlick PJ, Wernerman J. Protein-sparing effect in skeletal muscle of growth hormone treatment in critically ill patients. Ann Surg. 2000;231:577–586. doi: 10.1097/00000658-200004000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parry-Billings M, Evans J, Calder PC, Newsholme EA. Does glutamine contribute to immunosuppression after major burns. Lancet. 1990;336:523–525. doi: 10.1016/0140-6736(90)92083-t. [DOI] [PubMed] [Google Scholar]
- 23.Li N, DeMarco VG, West CM, Neu J. Glutamine supports recovery from loss of transepithelial resistance and increase of permeability induced by media change in Caco-2 cells. J Nutr Biochem. 2003;14:401–408. doi: 10.1016/s0955-2863(03)00071-8. [DOI] [PubMed] [Google Scholar]
- 24.Jung SE, Youn YK, Lim YS, Song HG, Rhee JE, Suh GJ. Combined administration of glutamine and growth hormone synergistically reduces bacterial translocation in sepsis. J Korean Med Sci. 2003;18:17–22. doi: 10.3346/jkms.2003.18.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye D, Ma I, Ma TY. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 26.Nylander O, Pihl L. Luminal hypotonicity increases duodenal mucosal permeability by a mechanism involving 5-hydroxytryptamine. Acta Physiol (Oxf) 2006;186:45–58. doi: 10.1111/j.1748-1716.2005.01507.x. [DOI] [PubMed] [Google Scholar]
- 27.Banan A, Zhang LJ, Farhadi A, Fields JZ, Shaikh M, Forsyth CB, Choudhary S, Keshavarzian A. Critical role of the atypical {lambda} isoform of protein kinase C (PKC-{lambda}) in oxidant-induced disruption of the microtubule cytoskeleton and barrier function of intestinal epithelium. J Pharmacol Exp Ther. 2005;312:458–471. doi: 10.1124/jpet.104.074591. [DOI] [PubMed] [Google Scholar]
- 28.Xu DZ, Lu Q, Deitch EA. Nitric oxide directly impairs intestinal barrier function. Shock. 2002;17:139–145. doi: 10.1097/00024382-200202000-00010. [DOI] [PubMed] [Google Scholar]


