Fig. 1.
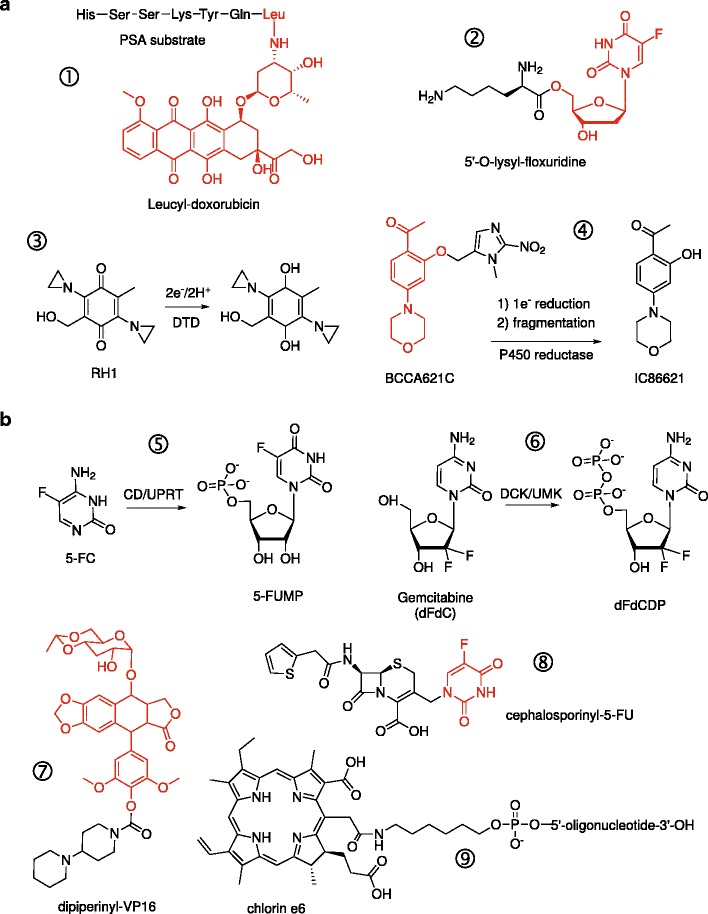
Passive and active conversion of prodrugs. Shown are illustrative examples of prodrugs that are activated by endogenous (passively) or exogenous (actively) enzymes, proteins, or conditions. In the case of conjugates, the active drug moiety is colored in red. a Examples of prodrugs that are substrates for endogenous proteases (① prostate-specific antigen, PSA) (8), membrane transporters (② PEPT1 oligopeptide transporter in pancreatic carcinomas) (14), or intracellular reductases (③ DT-diaphorase and ④ NADPH:cytochrome P450 reductase). b Prodrugs requiring exogenously administered enzymes or energy for activation. Activation of 5-fluorocytosine (⑤ 5-FC) and gemcitabine (⑥ dFdC) by engineered chimeric enzymes to their first cytotoxic antimetabolites. “Designer” conjugates of cytotoxic compounds as substrates for specific exogenous enzymes: ⑦ a recombinant carboxylesterase for dipiperinyl-VP-16 (40) and ⑧ β-lactamase for cephalosporinyl-5-FU (41). ⑨ A conjugate of the photosensitizer chlorin e6 with a single-stranded DNA aptamer that targets epithelial cancers presenting hypo-glycosylated MUC1 antigens (20). Irradiation at 664 nm generates cytotoxic singlet oxygen
