Abstract
Ambient particulate matter (PM) exposure has been associated with atherosclerosis. However, research on the effect of real-world exposure to ambient PM in regulating visfatin expression in an animal model is very limited. The objective is to investigate whether Beijing ambient PM exposure could accelerate atherosclerosis in ApoE knockout (ApoE−/−) mice by upregulating visfatin expression. Forty male ApoE−/− mice were exposed to untreated ambient air (PM group, n = 20) or filtered air (FA group, n = 20), 24 h/day, 7 days/week, for 2 months. During the exposure, the mass concentrations of PM2.5 and PM10 in the two groups were continuously monitored. Moreover, a receptor source apportionment model was applied to apportion sources of PM2.5. At the end of the exposure, visfatin in plasma and aorta, biomarkers of inflammation, oxidative stress and lipid metabolism in blood samples, and bronchoalveolar lavage fluid (BALF) were determined, and the plaque area of the atherosclerosis lesions was quantified. PM-exposed mice were significantly higher than FA-exposed mice in terms of plasma visfatin, OxLDL, MDA, serum TC, LDL, TNF-α as well as IL-6, TNF-α, OxLDL, and MDA in BALF, while SOD and GSH-Px activities in plasma and BALF were reduced in PM-exposed mice. Pathological analysis of the aorta demonstrated that the plaque area and visfatin protein in the PM group increased significantly compared to the FA group. Our findings indicate that ambient PM exposure could accelerate atherosclerosis, which is related to visfatin upregulation, as well as the activation of inflammation and oxidative stress.
Keywords: Particulate matter, Atherosclerosis, Visfatin, Systemic inflammation, Oxidative stress
Introduction
Air pollution is a pervasive environmental health hazard that occurs over a lifetime of exposure in individuals from many industrialized societies. Early epidemiological studies have shown that increased levels of air pollutants augment cardiovascular morbidity and mortality (Hoek et al. 2001). The first scientific statement on the association between air pollution and cardiovascular disease (CVD) was published by the American Heart Association (AHA) in 2004. Both gaseous and PM air pollutants have been found to have adverse effects on human health. PM initially appeared as the most detrimental, becoming the most investigated of pollutants with particular interest in CVD (Franchini and Mannucci 2012). Animal studies have demonstrated that chronic exposure to inhalable fine particles contributes to atherogenesis, the underlying pathology for cardiovascular events (Sun et al. 2008). Although numerous cross-sectional studies have reported an association between chronic exposure to PM and the development of atherosclerosis in humans (Adar et al. 2013; Hajat et al. 2013; Mani et al. 2013; Sun et al. 2013), some uncertainties still remain regarding the health effect in humans, possible multiple exposure to ambient PM, and exposure misclassification.
Visfatin [also termed pre-B cell colony-enhancing factor (PBEF) or nicotinamide phosphoribosyltransferase (Nampt)] is an adipokine, and early studies have mainly focused on the metabolic-associated diseases (such as diabetes and obesity) (Allen et al. 2012; Dogru et al. 2007). With the discovery of the pro-inflammatory role of visfatin, its potential effect in inflammation has gradually attracted much attention, especially in atherosclerosis, which is closely related to both lipid metabolism and inflammation (Liu et al. 2009; Yan et al. 2010). Enhanced visfatin content has been detected in human unstable atherosclerotic plaques (Dahl et al. 2007). Serum visfatin is increased in patients with carotid plaques (Zhong et al. 2008). Visfatin appears to be a direct contributor to vascular inflammation, a key feature of atherosclerosis (Romacho et al. 2009). In human endothelial cells, visfatin promotes nuclear factor-κB (NF-κB) activation, leading to the release of interleukin-6 (IL-6) and monocyte chemotactic protein-1 (MCP-1), the activation of matrix metalloproteinases (MMP-2, MMP-9), and the expression of vascular adhesion molecules (Adya et al. 2008; Adya et al. 2008; Liu et al. 2009). As a visfatin inhibitor, FK866 could reduce intraplaque chemokine (C-X-C motif) ligand 1 (CXCL1) production and associated neutrophil infiltration in atherosclerotic mice (Nencioni et al. 2013). Both visfatin and PM exposure are closely related to atherosclerosis, and although it has been reported that PM exposure plays a part in the pathogenesis of atherosclerosis (Araujo et al. 2008; Sun et al. 2008), the association between ambient PM exposure and regulating visfatin expression is largely unknown. It is important to investigate this association in order to contribute to a better understanding of the underlying biological mechanisms for PM-induced atherosclerosis.
Beijing, the capital city of China, is an international metropolis with a population of over 19 million. As in many big cities worldwide, air pollution is a major concern for city residents due to the very high population density, rapid increase in vehicular traffic, huge coal consumption during the winter heating season (November to March), and limited emissions controls. PM with an aerodynamic diameter less than 10 μm (PM10) or 2.5 μm (PM2.5) are the two main PM pollutants. The sources of PM10 consist of smoke, dirt, and dust from factories, farming and roads, as well as mold, spores, and pollen. PM2.5 is linked to toxic organic compounds, heavy metals (from smelting, processing, and others), the burning of plant material, and forest fires.
The level of PM is very low in the developed countries, and virtual impactor systems are used to deliver concentrated ambient particles (CAPs) and mimic exposure to high levels of “real-world” ambient air particles without the resuspension of artificial particles or invasive procedures. In most controlled exposure studies, subjects were exposed to different size fractions of PM of concentrated ambient air or aerosolized particles in a chamber. Changes in chemical composition of PM may alter its impact on human health. In order to simulate real-world PM exposure without changing the physicochemical property of PM, we decided to use non-concentrated real-world ambient PM in an animal whole body exposure chamber system in this study.
Although ambient PM exposure has been associated with atherosclerosis, it is still unknown whether inhalable PM accelerates the development of atherosclerosis by inducing the release of pro-inflammatory mediator visfatin leading to a local inflammatory response. The objective of this study was to investigate the association between Beijing ambient PM inhalation and visfatin expression in ApoE−/− mice.
Methods
Reagents
Rabbit antihuman visfatin (Peprotech, USA), goat anti-rabbit IgG antibody (Cell Signaling, USA), biotinylated goat anti-rabbit IgG (Vector Laboratories, USA), visfatin ELISA Kit (Raybiotech, USA), tumor necrosis factor-alpha (TNF-α) Kit, IL-6 Kit (eBioscience, USA), oxidized low-density lipoprotein (OxLDL) Kit (R&D Systems, Minneapolis, MN), superoxide dismutase (SOD) kit, glutathione peroxidase (GSH-Px) kit, malonaldehyde (MDA) kit (Nanjing Jiancheng Bioengineering Institute, China), BCA protein Assay Kit (Invitrogen, USA), total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) Reagent Kits (Invitrogen, USA).
Animal subjects
Apolipoprotein E (ApoE) is a ligand for receptors that clear low-density lipoproteins and remnants of chylomicrons. ApoE−/− mice developed foam cell-rich depositions in their aortas and had five times the normal plasma cholesterol (Zhang et al. 1992). ApoE−/− mice are widely used as an animal model for atherosclerosis because of these spontaneous lesions. Eight-week-old male ApoE−/− (C57BL/6J background) mice were obtained from Beijing Vital River Experimental Animal Technology Co. Ltd. All the mice were housed at 24 ± 1 °C, with a 12-h light/dark cycle. The mice were fed with a western diet (21 % fat, 0.15 % cholesterol, Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences) with the intention of establishing an atherosclerosis model. All the mice were allowed access to food and water ad libitum and were weighed each week. All animal experiments were carried out in accordance with international guidelines and were approved by the Ethics Committee of Southern Medical University.
Exposure to ambient air PM
Forty mice were randomly assigned into two groups. One was treated with untreated ambient air (PM group, n = 20) in a chamber, and one was supplied with ambient air filtered through a high-efficiency particulate air (HEPA) filter (FA group, n = 20) in the inlet valve position to remove most of the particles from the filtered airstream in another identical chamber. Exposure started at the age of 9 weeks, and 1 week was allowed for acclimation before exposure started. Mice were exposed for 24 h a day and 7 days a week for a total of 2 months from January 15 to March 15 in 2013. The two chambers were placed on the second floor of a building on the 4th Ring Road which is a major artery around the centre of Beijing, carrying 220,000 vehicles per day in eight main lanes and six auxiliary lanes (Duan et al. 2012).
A four-channel sampler (TH-16A, Wuhan, China; 16.70 L/min; 3 channels for 47 mm quartz filter and 1 channel for 47 mm Teflon filter) was used for the collection of filter-based samples for PM2.5. Quartz filters and Teflon filters were used respectively for organic and metal component analysis. The ambient PM2.5 and PM10 concentrations were measured continuously by TEOM 1405 (Thermo, USA) for comparison with the Dust Trak II Aerosol Monitor 8532 (TSI, USA) used in the PM chamber. The PM2.5 concentrations in the two chambers were measured by the same type of equipment.
Source apportionment
A receptor source apportionment model of chemical mass balance (US EPA CMB8.2) was used in the present study to apportion sources of PM2.5. This receptor model was constructed based on the hypothesis that inert tracers will keep mass balances during the transferring process between the sources and the receptor (ambient air). The details of the method were reported as previously described (Wei et al. 2010).
Serum lipids measurement
All mice were sacrificed after being fed on a western diet for 2 months. Mice were anesthetized by intraperitoneal injection with 10 % chloral hydrate (350 mg/kg), following eyeball extirpation to collect their blood. Serum and plasma were obtained after centrifugation of the collected blood samples at 4,000 rpm for 10 min and were stored at −80 °C until analysis. Serum concentrations of TC, TG, HDL, and LDL were determined by enzymatic procedures.
Biochemical analysis
To determine the circulating levels of plasma visfatin, serum TNF-α and IL-6, mice plasma, and serum were prepared and then analyzed using an enzyme-linked immunosorbent assay (ELISA) method according to the operating instructions of visfatin ELISA kit, TNF-α ELISA kit, and IL-6 ELISA kit. Plasma OxLDL was analyzed using ELISA kits as a marker of oxidative stress. The activities of GSH-Px and SOD and the contents of MDA were assayed by a commercial colorimetric assay kit.
Western blotting
The mice aortas were lysed in a lysis buffer containing 50 mM Tris (pH = 7.4), 150 mM NaCl, 1 % NP-40, and 1 mM PMSF for 30 min on ice. After centrifugation at 12,000g for 15 min at 4 °C, the supernatants were collected as total proteins in the aortas. The protein concentrations were also determined with a BCA assay kit. Aliquots (50 μg) of protein samples were separated on 10 % SDS-PAGE and electro-transferred to polyvinylidene fluoride membranes (PVDF, Millipore, Bedford, MA). The PVDF membranes were incubated with Rabbit antihuman visfatin overnight at 4 °C after being blocked with 5 % nonfat milk, and then subjected to Goat anti-rabbit IgG antibody for 1 h at 37 °C. The protein bands were detected with an enhanced chemiluminescence system (ECL, CST) on Kodak 2000MM. Densitometric analysis was conducted by Molecular Imaging Software Version 4.0. Actin proteins were detected as a control.
BALF analysis
Bronchoalveolar lavage fluid (BALF) was used to assess pulmonary inflammation response and oxidative stress. After the collection of blood samples, the lung and trachea were exposed by dissection, and the left lung was temporarily clamped. The right lung was lavaged with 6 ml of warm normal saline, the recovered BALF were centrifuged at 400×g for 10 min, and the collected supernatant was analyzed for IL-6, TNF-α, OxLDL, SOD, GSH-Px, and MDA by ELISA assay. Each sample was tested in duplicate.
Plaque area quantification
The aortic tree was perfusion-fixed at a constant, near-physiological pressure via a cannula inserted in the right ventricle, allowing unrestricted efflux from an incision in the left atrium. Blood was removed by perfusion with 25 ml of phosphate-buffered saline (PBS, 10 Mm, pH = 7.4) followed by 40 ml of 4 % buffered paraformaldehyde (pH = 7.4) to obtain an initial fixation. The aorta was harvested and fixed in 10 % zinc-formalin embedded in paraffin and cross-sectioned at 5 μm for hematoxylin and eosin (H&E) staining. Atherosclerotic lesions in the aortic root were examined at three locations and each was separated by 80 μm; three serial sections were prepared from each location. All images were captured with a camera and analyzed using ImageJ software (National Institutes of Health) to quantify the area of the atherosclerosis lesions.
Immunohistochemistry
To identify the distribution of visfatin in the atherosclerotic plaques, we measured its levels in the atherosclerotic lesions. After antigen retrieval for 10 min by boiling in 0.01 M sodium citrate, deparaffinized sections were quenched in 0.3 % hydrogen peroxide for 30 min and incubated in 1 % BSA in PBS for 30 min. Sections were incubated with rabbit antihuman visfatin (10 ng/ml) overnight at 4 °C. Samples were detected with biotinylated goat anti-rabbit IgG diluted 1:1,000 for 30 min, washed, followed by streptavidin peroxidase for 30 min, and color visualized using 3, 3′-diaminobenzidine (DAB) for brown color. The sections were counterstained with hematoxylin and examined by light microscopy.
Statistical analysis
SPSS 13.0 was used for data analysis. Data are presented as mean ± SD. A student’s T test was used for comparing the two groups, and one-way analysis of variance was used for more than two groups. P < 0.05 was considered statistically significant.
Results
Animal subjects
There were no baseline differences in weight between the two groups at the beginning of the exposure. At the time of sacrifice, weight increased in all mice compared with the baseline (Table 1).
Table 1.
Body weight (BW) and lipid measurements in ApoE−/− mice after 2 months of FA and PM inhalation
| Group | Body weight(BW, g) | Lipid measurements | ||||
|---|---|---|---|---|---|---|
| Start | End | TC (mmol/L) | TG (mmol/L) | HDL (μmol/L) | LDL (μmol/L) | |
| FA | 24.98 ± 1.47 | 31.79 ± 2.26 | 35.32 ± 4.21 | 17.42 ± 5.16 | 76.08 ± 2.26 | 119.38 ± 10.34 |
| PM | 25.23 ± 1.72 | 32.18 ± 2.19 | 44.23 ± 4.93* | 18.76 ± 5.22 | 77.35 ± 2.41 | 134.42 ± 11.41* |
Values are mean ± SD (n = 20/group)
*P < 0.05 vs FA group
Concentrations of PM2.5 and PM10
During the exposure, the daily PM2.5 concentrations (mean ± SD) in the FA chamber and the PM chamber were 19.2 ± 11.7 and 63.1 ± 67.4 μg/m3, respectively, and the daily PM10 concentration in the PM chamber was 100.3 ± 71.2 μg/m3. The concentration of PM10 in the FA chamber was close to that of PM2.5 due to the utilization of a HEPA filter by which most particles (>98 %) with an aerodynamic diameter larger than 2.5 μm were filtered and removed before entering the FA chamber. In our study, we found that the PM2.5 concentration in the PM chamber was much higher than the National Ambient Air Quality Standards (NAAQS, 2012) set by the US Environmental Protection Agency (EPA) (35 μg/m3 for 24 h mean) and the Global Air Quality Guidelines set by the World Health Organization in 2005 (25 μg/m3 for 24 h mean).
Source apportionment analysis
In the present paper, we used CMB to identify potential sources of PM2.5 using metal and organic tracers. The CMB model identified seven sources for PM2.5 exposure samples and explained about 80 % of the total mass of PM2.5 (Fig. 1). Among the estimated seven sources, gasoline vehicles (38.7 %) and coal burning (25.1 %) emissions accounted for almost two thirds of the total mass concentration of PM2.5.
Fig. 1.
The source apportionments of PM2.5. Gasoline vehicles and coal burning emissions were the two major resources of PM2.5
Lipid metabolism
After 2 months of PM exposure, the levels of serum lipids such as TC and LDL increased significantly more in mice exposed to PM than in the FA group, while PM exposure did not alter TG and HDL levels compared with the FA group (Table 1).
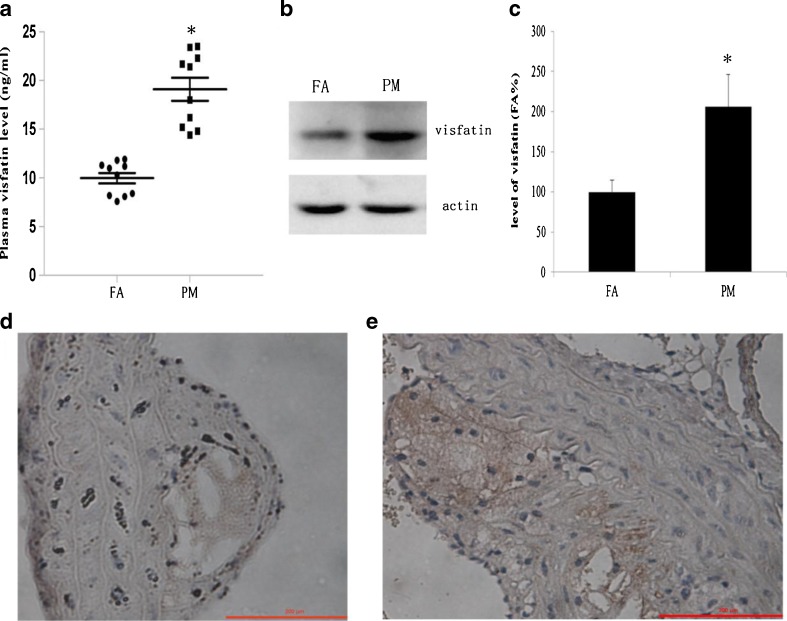
PM exposure upregulated the expression of visfatin
To analyze visfatin expression in a mouse atherosclerosis model, we determined its level in both plasma and atherosclerotic lesions by ELISA, immunohistochemistry, and Western blotting assay, respectively. We found that both plasma visfatin levels and visfatin protein in the aorta in the PM group were much higher than in the FA group. The plasma visfatin level in the PM group was almost 1.93 times that in the FA group (Fig. 2a). Western blotting assay showed that the protein level of visfatin in the aorta of the PM group was almost 1.91 times that of the FA group (Fig. 2c). Visfatin measured in the aorta by immunohistochemical staining showed that significantly more visfatin was detected from mice exposed to PM compared with the FA mice (Fig. 2d, e). These results indicate that ambient PM may play a role in upregulating the expression of visfatin and in the pathogenesis of atherosclerosis.
Fig. 2.
Visfatin expression after 2 months of FA and PM inhalation. a Plasma levels of visfatin in ApoE−/− mice were determined by ELISA (n = 10). *P < 0.05 vs FA group. b The protein expression of visfatin in mice aorta was determined by Western blot analysis. c Protein bands of visfatin and actin were quantified by Molecular Imaging Software (n = 3). *P < 0.05 vs FA group. d Representative image of immunohistochemistry for visfatin in mice aorta from FA group. e Representative image of immunohistochemistry for visfatin in mice aorta from PM group. Increased visfatin staining (brown) is shown in PM-exposed mice compared to FA-exposed mice (magnification ×200)
PM exposure accelerated the progression of atherosclerosis
We measured plaque area using cross-sectional slices of the ascending aorta to evaluate the progression of atherosclerosis. Representative H&E stained cross-sections were shown (Fig. 3a, b). At all locations of the ascending aorta, PM mice had a significantly larger lesion area than that of FA mice, and plaque was observed predominantly in the intimal and medial areas of the arterial wall and less so in the adventitial layers (Fig. 3c).
Fig. 3.
Atherosclerotic lesions in mice aorta after 2 months of FA and PM inhalation. a Representative H&E-stained cross-sections of aorta in mice from FA group (magnification ×40). b Representative H&E-stained cross-sections of aorta in mice from PM group. c Plaque area at aorta in the two groups. Values are mean ± SD (n = 20/group). *P < 0.05 vs FA group
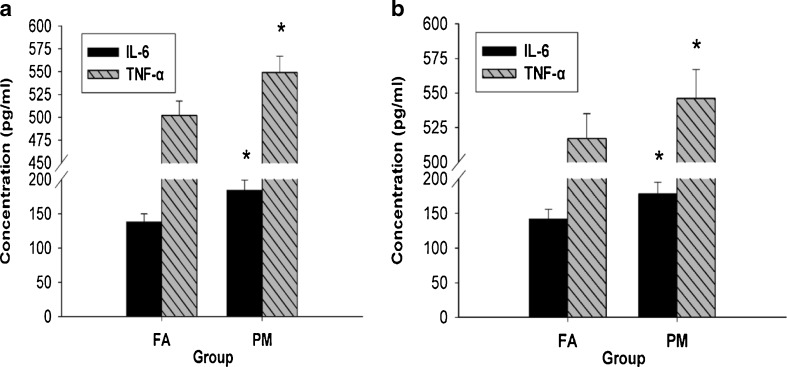
PM exposure activated systemic inflammation and pulmonary inflammation
We assessed TNF-α and IL-6 in both serum and BALF in order to define the systemic inflammation and pulmonary inflammation responses, respectively. We found that both TNF-α and IL-6 in serum of the PM group was significantly higher than in that of the FA group (Fig. 4a). We also found a significant increase in TNF-α and IL-6 levels in BALF of the PM group, which indicated persistent inflammation induced by PM exposure (Fig. 4b).
Fig. 4.
System inflammation and pulmonary inflammation after 2 months of FA and PM inhalation. a Serum levels of TNF-α and IL-6 in ApoE−/− mice were determined by ELISA. b BALF levels of TNF-α and IL-6 in ApoE−/− mice were determined by ELISA. Values are mean ± SD (n = 10/group). *P < 0.05 vs FA group
PM exposure-induced oxidative stress
Oxidative stress has been regarded as a common pathogenic mechanism in atherosclerosis. Plasma and BALF OxLDL levels increased significantly more in mice exposed to PM than in the FA group (Fig. 5a, d). The contents of MDA in plasma and BALF increased significantly in PM-exposed mice compared to FA-exposed mice (Fig. 5c, f), while SOD and GSH-Px activities in plasma and BALF were reduced in PM-exposed mice compared to FA-exposed mice (Fig. 5b, e).
Fig. 5.
Oxidative stress status after 2 months of FA and PM inhalation. a Plasma levels of OxLDL in ApoE−/− mice were determined by ELISA. b Plasma levels of SOD and GSH-Px in ApoE−/− mice. c Plasma levels of MDA in ApoE−/− mice. d BALF levels of OxLDL in ApoE−/− mice. e BALF levels of SOD and GSH-Px in ApoE−/− mice. f BALF levels of MDA in ApoE−/− mice. Values are mean ± SD (n = 10/group). *P < 0.05 vs FA group, **P < 0.01 vs FA group
Discussion
This study is the first to investigate the association between untreated Beijing ambient air exposure and regulating visfatin expression in the development of atherosclerosis in ApoE−/− mice. The results of the study can be used to explain the long-term impact of PM exposure on susceptible human populations. Our data indicate that long-term PM exposure correlates with the progression of atherosclerosis, which is important in explaining how ambient PM contributes to CVD.
Visfatin, previously defined as PBEF, is a 52-kDa cytokine expressed and secreted by lymphocytes (Samal et al. 1994). Visfatin is also called Nampt because of its functional and biochemical homology with nicotinamide adenine dinucleotide (NAD) biosynthesis from nicotinamide (Rongvaux et al. 2002). The first report of visfatin being an adipokine and preferentially produced by visceral adipose tissue indicated that visfatin activated the insulin receptor in 2005 (Fukuhara et al. 2005). However, the expectation initially arose by the novel adipokine was soon blunted when the authors had to retract their paper due to the lack of reproducibility of the hypoglycemic properties (Fukuhara et al. 2007). Although the exact role of visfatin in the development of atherosclerosis remains to be elucidated, a critical role in pathobiology is suggested by its high degree of conservation throughout evolution. Visfatin appears to play an important role as a cytokine secreted extracellularly in response to inflammatory stimuli such as TNF-α, IL-1β, and IL-6 (Moschen et al. 2007). Visfatin expression is increased by plaque macrophages in patients with unstable carotid and coronary atherosclerosis (Dahl et al. 2007). Our research group have recently reported that visfatin promotes lipid accumulation mainly through excessive cholesterol uptake in RAW264.7 macrophages and in peritoneal macrophages isolated from ApoE−/− mice and accelerates the process of atherosclerosis mainly through modulating the expression of the macrophage scavenger receptor class A (SR-A) and CD36 (Zhou et al. 2013). Visfatin effects on cytokine and chemokine secretion, macrophage survival, vascular smooth muscle inflammation, and plaque destabilization make of this adipokine an active factor in the development of atherosclerosis. However, the effect of PM exposure and its possible mechanism involved in visfatin expression in the development and progression of atherosclerosis remained elusive. In this study, we found that exposure to Beijing ambient PM resulted in upregulation of visfatin levels in both plasma and atherosclerotic lesions, and these data suggest that visfatin would play an important role in atherogenesis during PM exposure. Further prospective studies will be necessary to clarify this issue because we did not assess clinical outcomes in the present study.
The most prominent hypothesis on the mechanism relating air pollution to cardiorespiratory mortality and morbidity has been that air pollutants, especially fine and ultrafine particles, provoke pulmonary inflammation, largely by induction of oxidative stress. This oxidative stress then may lead to increased blood coagulability and systemic inflammation over a matter of hours to days (Seaton et al. 1999; Watt et al. 1995). The current theory of the oxidative modification hypothesis states that LDL becomes oxidized in the arterial wall where it then lends itself to cellular uptake and foam cell formation. It is said that SOD and GSH-Px are the two key antioxidant enzymes in the body, which help free radicals to react with other chemicals to produce safe instead of toxic substances (Perez et al. 2013), and that MDA, a marker of lipid peroxidation and oxidative stress, damages cells and tissues. Research has demonstrated that inflammation and oxidative stress initiated by reactive oxygen species (ROS) within affected cells were involved in adverse effects on animals (Romieu et al. 2008). PM can generate ROS both directly and indirectly; PM incubation has been suggested to activate ROS-generating pathways in both pulmonary and vascular tissues (Barregard et al. 2006; Li et al. 2006). Plasma OxLDL was used as the biomarker of early atherosclerosis and it was associated with chronic exposure to air pollution (Jacobs et al. 2011). OxLDL-induced endothelial damage, monocyte adhesion, platelet aggregation, and inhibited apoptosis and endothelial nitric oxide synthase (eNOS) expression/activity, all of which contributed to atherosclerotic process (Li and Mehta 2003). Our findings of a significant reduction of SOD and GSH-Px and an increase of OxLDL and MDA levels in plasma and BALF of PM-exposed mice suggest that PM is vital to oxidative stress. Interactions between PM exposure and oxidative stress may therefore be crucial to the effects observed in our study, and such interactions may help to explain effects of PM-related morbidity and mortality in a susceptible population.
It is possible that an inflammatory response in the lung (cells and circulating mediators) may result in activation of inflammatory cascades in the vessel wall and potentiation of atherogenesis. Numerous inflammatory mediators that are released from lung cells after exposure to PM may spread to general circulation, where they can plausibly modulate systemic effects. Several inflammatory cytokines, such as interferon-γ, TNF-α, IL-1β, and IL-6, have been shown to be increased in circulating blood, as well as in bronchial fluid after PM inhalation in both human and animal studies (Hartz et al. 2008; Mutlu et al. 2011; Tornqvist et al. 2007). PM-induced vascular inflammation may have adverse effects also on metabolic pathways. Mice exposed long-term to PM2.5 exhibited marked whole body insulin resistance, increased visceral adiposity and visceral adipose inflammation concomitant with vascular relaxation abnormalities, and enhanced monocyte adhesion to microcirculatory beds (Sun et al. 2009). Ultrafine PM may directly enter cells via non-phagocytic pathways and then impair organelles (such as mitochondria) because of their nanoscale size (Moller et al. 2010; Muhlfeld et al. 2008). Mitochondrial DNA copy number (MtDNAcn), as a marker of mitochondrial damage and malfunctioning, was negatively associated with increased exposure to elemental carbon during work hours and recent ambient PM10 exposure (Hou et al. 2013). Larger PM enters the cells by phagocytosis through interactions with innate immunity receptors, such as macrophage receptor with scavenger receptors or collagenous structure (Moller et al. 2010; Muhlfeld et al. 2008). Moreover, acute exposure of coarse PM has been shown to directly trigger inflammation by binding to toll-like receptor (TLR) 2 and 4, and PM2.5 can drive a Th-2-biased immune response (Zhao et al. 2012).
Although the findings of this study could give new insights to the understanding of the increase in cardiopulmonary morbidity and mortality in association with PM exposure, other mechanisms still play important roles in PM-induced atherogenesis, such as human bronchial cell cycle alteration and NO-mediated endothelial dysfunction (Cachon et al. 2014; Krishnan et al. 2012). Further studies are needed to explore the mechanism from increased visfatin induced by PM exposure.
Conclusions
In summary, our findings show that inhalation exposure to Beijing ambient PM results in upregulation of the potential inflammatory mediator, visfatin. The effect of enhanced atherogenic response in ApoE−/− mice could be linked to its ability to generate inflammation and oxidative stress. In this study, we decided to use non-concentrated real-world ambient PM instead of concentrated PM to reveal that real ambient PM exposure has a strong proatherogenic effect. Our findings provide a potential biological basis for the association between atherosclerosis-related events and prospective population cohort studies. As atherosclerosis is the primary underlying pathological determinant of the onset of acute myocardial infarction (AMI) and stroke, mechanisms that drive upregulation of visfatin, in response to PM exposure, require further investigation and clarification to determine effective prevention and treatment options.
Acknowledgments
This project was supported by grants from the National Natural Sciences Foundation of China (no. 81072776 and no. 81373574).
Conflict of interest
None.
Footnotes
Qiang Wan and Xiaobing Cui contributed equally to this work.
Contributor Information
Fenghua Zhou, Phone: +86-20-61648767, FAX: +86-20-61648767, Email: wendyzhou515@126.com.
Yuhua Jia, Phone: +86-20-61648250, FAX: +86-20-61648250, Email: yhjia_smu@163.com.
References
- Adar SD, Sheppard L, Vedal S, Polak JF, Sampson PD, Diez Roux AV, Budoff M, Jacobs DR, Jr, Barr RG, Watson K, Kaufman JD. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the multi-ethnic study of atherosclerosis and air pollution. PLoS Med. 2013;10:e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adya R, Tan BK, Chen J, Randeva HS. Nuclear factor-kappaB induction by visfatin in human vascular endothelial cells: its role in MMP-2/9 production and activation. Diabetes care. 2008;31:758–760. doi: 10.2337/dc07-1544. [DOI] [PubMed] [Google Scholar]
- Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78:356–365. doi: 10.1093/cvr/cvm111. [DOI] [PubMed] [Google Scholar]
- Allen RW, Adar SD, Avol E, Cohen M, Curl CL, Larson T, Liu LJ, Sheppard L, Kaufman JD. Modeling the residential infiltration of outdoor PM(2.5) in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Health Perspect. 2012;120:824–830. doi: 10.1289/ehp.1104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res. 2008;102:589–596. doi: 10.1161/CIRCRESAHA.107.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barregard L, Sallsten G, Gustafson P, Andersson L, Johansson L, Basu S, Stigendal L. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxic. 2006;18:845–853. doi: 10.1080/08958370600685798. [DOI] [PubMed] [Google Scholar]
- Cachon BF, Firmin S, Verdin A, Ayi-Fanou L, Billet S, Cazier F, Martin PJ, Aissi F, Courcot D, Sanni A, Shirali P. Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM2.5 and PM > 2.5) collected from Cotonou, Benin. Environ Pollut. 2014;185:340–351. doi: 10.1016/j.envpol.2013.10.026. [DOI] [PubMed] [Google Scholar]
- Dahl TB, Yndestad A, Skjelland M, Oie E, Dahl A, Michelsen A, Damas JK, Tunheim SH, Ueland T, Smith C, Bendz B, Tonstad S, Gullestad L, Froland SS, Krohg-Sorensen K, Russell D, Aukrust P, Halvorsen B. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: possible role in inflammation and plaque destabilization. Circulation. 2007;115:972–980. doi: 10.1161/CIRCULATIONAHA.106.665893. [DOI] [PubMed] [Google Scholar]
- Dogru T, Sonmez A, Tasci I, Bozoglu E, Yilmaz MI, Genc H, Erdem G, Gok M, Bingol N, Kilic S, Ozgurtas T, Bingol S. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res Clin Pract. 2007;76:24–29. doi: 10.1016/j.diabres.2006.07.031. [DOI] [PubMed] [Google Scholar]
- Duan J, Tan J, Wang S, Hao J, Chai F. Size distributions and sources of elements in particulate matter at curbside, urban and rural sites in Beijing. J Environ Sci. 2012;24:87–94. doi: 10.1016/S1001-0742(11)60731-6. [DOI] [PubMed] [Google Scholar]
- Franchini M, Mannucci PM. Air pollution and cardiovascular disease. Thromb Res. 2012;129:230–234. doi: 10.1016/j.thromres.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Retraction. Science. 2007;318:565. doi: 10.1126/science.318.5850.565b. [DOI] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux AV, Adar SD, Auchincloss AH, Lovasi GS, O’Neill MS, Sheppard L, Kaufman JD (2013) Air pollution and individual and neighborhood socioeconomic status: evidence from the Multi-Ethnic Study of Atherosclerosis (MESA). Environmental health perspectives [DOI] [PMC free article] [PubMed]
- Hartz AM, Bauer B, Block ML, Hong JS, Miller DS. Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB J: Off Publ Fed Am Soc Exp Biol. 2008;22:2723–2733. doi: 10.1096/fj.08-106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Brunekreef B, Fischer P, van Wijnen J. The association between air pollution and heart failure, arrhythmia, embolism, thrombosis, and other cardiovascular causes of death in a time series study. Epidemiology. 2001;12:355–357. doi: 10.1097/00001648-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Hou L, Zhang X, Dioni L, Barretta F, Dou C, Zheng Y, Hoxha M, Bertazzi PA, Schwartz J, Wu S, Wang S, Baccarelli AA. Inhalable particulate matter and mitochondrial DNA copy number in highly exposed individuals in Beijing, China: a repeated-measure study. Part Fibre Toxic. 2013;10:17. doi: 10.1186/1743-8977-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L, Emmerechts J, Hoylaerts MF, Mathieu C, Hoet PH, Nemery B, Nawrot TS. Traffic air pollution and oxidized LDL. PloS one. 2011;6:e16200. doi: 10.1371/journal.pone.0016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, O’Neill MS, Herrington DM, Polak JF, Kaufman JD. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution) J Am Coll Cardiol. 2012;60:2158–2166. doi: 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mehta JL. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect against oxidized low-density lipoprotein-induced endothelial dysfunction. Endothelium: J Endothelial Cell Res. 2003;10:17–21. doi: 10.1080/10623320303355. [DOI] [PubMed] [Google Scholar]
- Li Z, Hyseni X, Carter JD, Soukup JM, Dailey LA, Huang YC. Pollutant particles enhanced H2O2 production from NAD(P)H oxidase and mitochondria in human pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2006;291:C357–C365. doi: 10.1152/ajpcell.00365.2005. [DOI] [PubMed] [Google Scholar]
- Liu SW, Qiao SB, Yuan JS, Liu DQ. Association of plasma visfatin levels with inflammation, atherosclerosis and acute coronary syndromes (ACS) in humans. Clin Endocrinol. 2009;71:202–207. doi: 10.1111/j.1365-2265.2008.03453.x. [DOI] [PubMed] [Google Scholar]
- Liu SW, Qiao SB, Yuan JS, Liu DQ. Visfatin stimulates production of monocyte chemotactic protein-1 and interleukin-6 in human vein umbilical endothelial cells. Hormone Metab Res. 2009;41:281–286. doi: 10.1055/s-0028-1102914. [DOI] [PubMed] [Google Scholar]
- Mani V, Wong SK, Sawit ST, Calcagno C, Maceda C, Ramachandran S, Fayad ZA, Moline J, McLaughlin MA. Relationship between particulate matter exposure and atherogenic profile in “Ground Zero” workers as shown by dynamic contrast enhanced MR imaging. Int J Cardiovasc Imag. 2013;29:827–833. doi: 10.1007/s10554-012-0154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S. Role of oxidative damage in toxicity of particulates. Free Radical Res. 2010;44:1–46. doi: 10.3109/10715760903300691. [DOI] [PubMed] [Google Scholar]
- Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- Muhlfeld C, Rothen-Rutishauser B, Blank F, Vanhecke D, Ochs M, Gehr P. Interactions of nanoparticles with pulmonary structures and cellular responses. Am J Physiol Lung Cell Mol Physiol. 2008;294:L817–L829. doi: 10.1152/ajplung.00442.2007. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Engen PA, Soberanes S, Urich D, Forsyth CB, Nigdelioglu R, Chiarella SE, Radigan KA, Gonzalez A, Jakate S, Keshavarzian A, Budinger GR, Mutlu GM. Particulate matter air pollution causes oxidant-mediated increase in gut permeability in mice. Part Fibre Toxic. 2011;8:19. doi: 10.1186/1743-8977-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nencioni A, da Silva RF, Fraga-Silva RA, Steffens S, Fabre M, Bauer I, Caffa I, Magnone M, Sociali G, Quercioli A, Pelli G, Lenglet S, Galan K, Burger F, Vazquez Calvo S, Bertolotto M, Bruzzone S, Ballestrero A, Patrone F, Dallegri F, Santos RA, Stergiopulos N, Mach F, Vuilleumier N, Montecucco F (2013) Nicotinamide phosphoribosyltransferase inhibition reduces intraplaque CXCL1 production and associated neutrophil infiltration in atherosclerotic mice. Thrombosis and haemostasis 111 [DOI] [PubMed]
- Perez Y, Oyarzabal A, Mas R, Molina V, Jimenez S. Protective effect of D-002, a mixture of beeswax alcohols, against indomethacin-induced gastric ulcers and mechanism of action. J Nat Med. 2013;67:182–189. doi: 10.1007/s11418-012-0670-y. [DOI] [PubMed] [Google Scholar]
- Romacho T, Azcutia V, Vazquez-Bella M, Matesanz N, Cercas E, Nevado J, Carraro R, Rodriguez-Manas L, Sanchez-Ferrer CF, Peiro C. Extracellular PBEF/NAMPT/visfatin activates pro-inflammatory signalling in human vascular smooth muscle cells through nicotinamide phosphoribosyltransferase activity. Diabetologia. 2009;52:2455–2463. doi: 10.1007/s00125-009-1509-2. [DOI] [PubMed] [Google Scholar]
- Romieu I, Garcia-Esteban R, Sunyer J, Rios C, Alcaraz-Zubeldia M, Velasco SR, Holguin F. The effect of supplementation with omega-3 polyunsaturated fatty acids on markers of oxidative stress in elderly exposed to PM(2.5) Environ Health Perspect. 2008;116:1237–1242. doi: 10.1289/ehp.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, Andris F. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton A, Soutar A, Crawford V, Elton R, McNerlan S, Cherrie J, Watt M, Agius R, Stout R. Particulate air pollution and the blood. Thorax. 1999;54:1027–1032. doi: 10.1136/thx.54.11.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Yue P, Kirk RI, Wang A, Moatti D, Jin X, Lu B, Schecter AD, Lippmann M, Gordon T, Chen LC, Rajagopalan S. Ambient air particulate matter exposure and tissue factor expression in atherosclerosis. Inhal Toxic. 2008;20:127–137. doi: 10.1080/08958370701821482. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Kaufman JD, Kim SY, Larson TV, Gould TR, Polak JF, Budoff MJ, Diez Roux AV, Vedal S. Particulate matter components and subclinical atherosclerosis: common approaches to estimating exposure in a Multi-Ethnic Study of Atherosclerosis cross-sectional study. Environ Health: Global Access Sci Sourc. 2013;12:39. doi: 10.1186/1476-069X-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. doi: 10.1164/rccm.200606-872OC. [DOI] [PubMed] [Google Scholar]
- Watt M, Godden D, Cherrie J, Seaton A. Individual exposure to particulate air pollution and its relevance to thresholds for health effects: a study of traffic wardens. Occup Environ Med. 1995;52:790–792. doi: 10.1136/oem.52.12.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Han IK, Hu M, Shao M, Zhang JJ, Tang X. Personal exposure to particulate PAHs and anthraquinone and oxidative DNA damages in humans. Chemosphere. 2010;81:1280–1285. doi: 10.1016/j.chemosphere.2010.08.055. [DOI] [PubMed] [Google Scholar]
- Yan JJ, Tang NP, Tang JJ, Jia EZ, Wang MW, Wang QM, Zhu J, Yang ZJ, Wang LS, Huang J. Genetic variant in visfatin gene promoter is associated with decreased risk of coronary artery disease in a Chinese population. Clinica chimica acta Intern J Clin Chem. 2010;411:26–30. doi: 10.1016/j.cca.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- Zhao C, Liao J, Chu W, Wang S, Yang T, Tao Y, Wang G. Involvement of TLR2 and TLR4 and Th1/Th2 shift in inflammatory responses induced by fine ambient particulate matter in mice. Inhal Toxic. 2012;24:918–927. doi: 10.3109/08958378.2012.731093. [DOI] [PubMed] [Google Scholar]
- Zhong M, Tan HW, Gong HP, Wang SF, Zhang Y, Zhang W. Increased serum visfatin in patients with metabolic syndrome and carotid atherosclerosis. Clin Endocrin. 2008;69:878–884. doi: 10.1111/j.1365-2265.2008.03248.x. [DOI] [PubMed] [Google Scholar]
- Zhou F, Pan Y, Huang Z, Jia Y, Zhao X, Chen Y, Diao J, Wan Q, Cui X. Visfatin induces cholesterol accumulation in macrophages through up-regulation of scavenger receptor-A and CD36. Cell Stress Chaperones. 2013;18:643–652. doi: 10.1007/s12192-013-0417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]