Abstract
Quantitative analyses of cell walls from Streptococcus mutans Ingbritt grown under carbohydrate limitation in the chemostat showed that growth conditions had no statistically significant effect on the composition of polysaccharide, peptidoglycan, or the proportion of polysaccharide in the cell wall. Lysis of cell wall preparations with a muramidase supported this conclusion and further indicated that there was little difference in their overall structure. In contrast, there was a consistent difference between the rates of lysis by this enzyme of organisms grown in 0.2% glucose and 0.5% glucose. Extremes of pH or dilution rate essentially did not influence the immunogenicity of type c antigen in whole organisms irrespective of whether the carbohydrate source was glucose or sucrose. However, differences were found in the immunogenicity of lipoteichoic acid under similar circumstances. The results indicated there was an inherent phenotypic stability in the cell walls of S. mutans Ingbritt despite changes in pH, generation time, and carbohydrate source, and that any changes that did occur were probably due to associated cell-surface components.
Full text
PDF


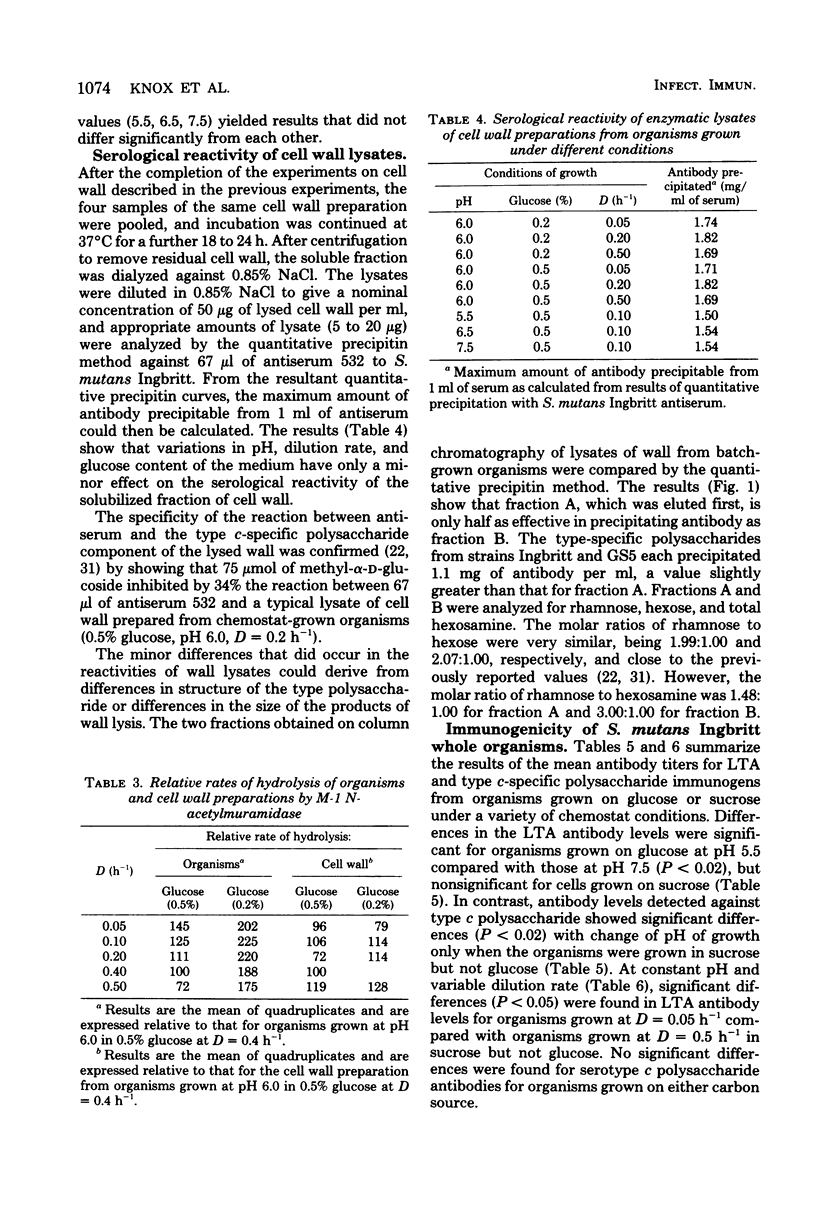
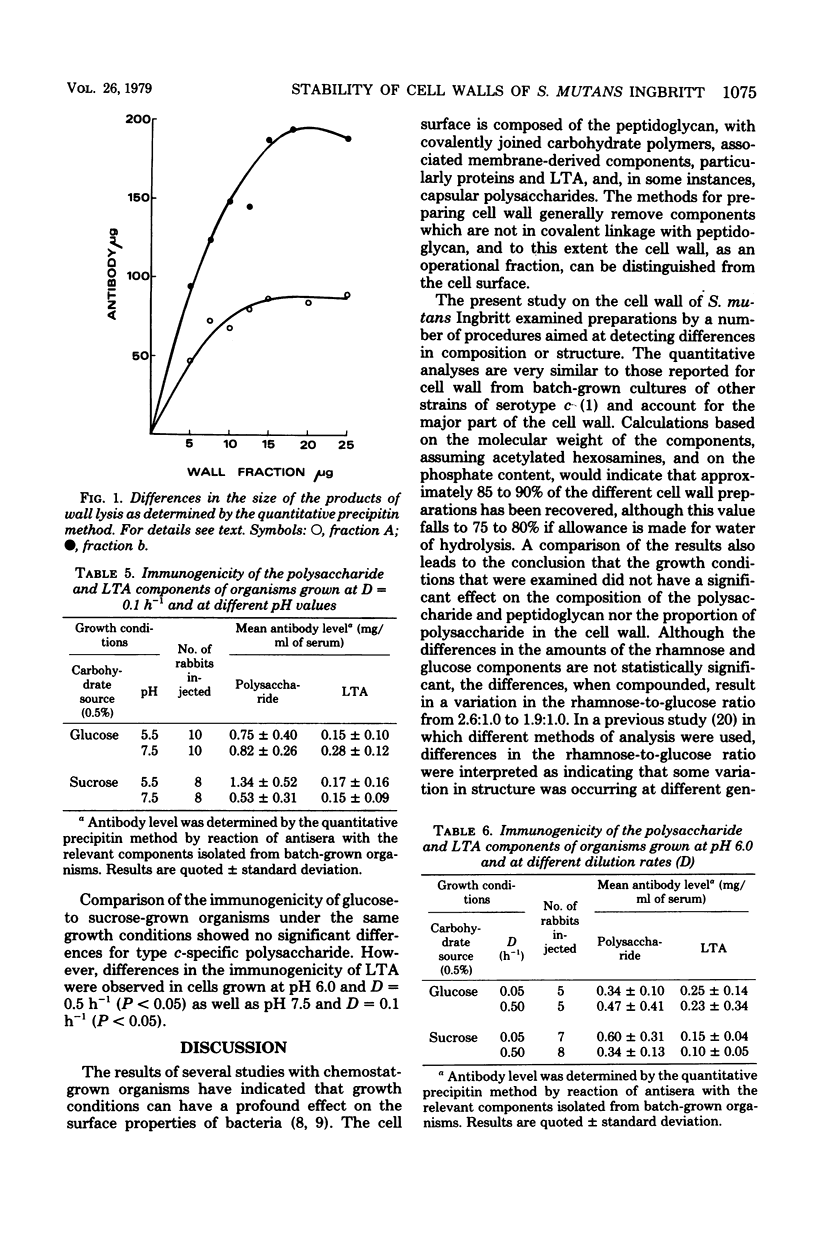



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleiweis A. S., Taylor M. C., Deepak J., Brown T. A., Wetherell J. R., Jr Comparative chemical compositions of cell walls of Streptococcus mutans. J Dent Res. 1976 Jan;55:A103–A108. doi: 10.1177/002203457605500102011. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of Streptococcus mutans strains in some selected areas of the world. Odontol Revy. 1972;23(4):401–410. [PubMed] [Google Scholar]
- Bratthall D. Immunodiffusion studies on the serological specificity of streptococci resembling Streptococcus mutans. Odontol Revy. 1969;20(3):231–243. [PubMed] [Google Scholar]
- Bratthall D., Pettersson B. M. Common and unique antigens of Streptococcus mutants. J Dent Res. 1976 Jan;55:A60–A64. doi: 10.1177/002203457605500123011. [DOI] [PubMed] [Google Scholar]
- Campbell L. K., Knox K. W., Wicken A. J. Extractability of cell wall polysaccharide from lactobacilli and streptococci by autoclaving and by dilue acid. Infect Immun. 1978 Dec;22(3):842–851. doi: 10.1128/iai.22.3.842-851.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- GIBBONS R. J. BACTERIOLOGY OF DENTAL CARIES. J Dent Res. 1964 Nov-Dec;43:SUPPL–SUPPL:1028. doi: 10.1177/00220345640430060301. [DOI] [PubMed] [Google Scholar]
- Hamada S., Torii M., Kotani S., Masuda N., Ooshima T., Yokogawa K., Kawata S. Lysis of Streptococcus mutans cells with mutanolysin, a lytic enzyme prepared from a culture liquor of Streptomyces globisporus 1829. Arch Oral Biol. 1978;23(7):543–549. doi: 10.1016/0003-9969(78)90268-6. [DOI] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Campbell L. K., Knox K. W., Evans J. D., Wicken A. J. Effect of carbohydrate source and growth conditions on the production of lipoteichoic acid by Streptococcus mutans Ingbritt. Infect Immun. 1979 Dec;26(3):1079–1087. doi: 10.1128/iai.26.3.1079-1087.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques N. A., Hardy L., Knox K. W., Wicken A. J. Effect of growth conditions on the formation of extracellular lipoteichoic acid by Streptococcus mutans BHT. Infect Immun. 1979 Jul;25(1):75–84. doi: 10.1128/iai.25.1.75-84.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Campbell J. N. Effect of growth conditions on peptidoglycan structure and susceptibility to lytic enzymes in cell walls of Micrococcus sodonensis. Biochemistry. 1972 Jan 18;11(2):277–286. doi: 10.1021/bi00752a020. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Campbell L. K., Broady K. W., Wicken A. J. Serological studies on chemostat-grown cultures of Lactobacillus fermentum and Lactobacillus plantarum. Infect Immun. 1979 Apr;24(1):12–18. doi: 10.1128/iai.24.1.12-18.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Hall E. A. The linkage between the polysaccharide and mucopeptide components of the cell wall of Lactobacillus casei. Biochem J. 1965 Aug;96(2):302–309. doi: 10.1042/bj0960302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Hewett M. J., Wicken A. J. Studies on the group F antigen of lactobacilli: antigenicity and serological specificity of teichoic acid preparations. J Gen Microbiol. 1970 Mar;60(3):303–313. doi: 10.1099/00221287-60-3-303. [DOI] [PubMed] [Google Scholar]
- Linzer R., Gill K., Slade H. D. Chemical composition of Streptococcus mutans type c antigen: comparison to type a, b, and d antigens. J Dent Res. 1976 Jan;55:A109–A115. doi: 10.1177/002203457605500103011. [DOI] [PubMed] [Google Scholar]
- Pearson A. D., Ellwood D. C. Growth environment and bacterial toxicity. J Med Microbiol. 1974 Aug;7(3):391–393. doi: 10.1099/00222615-7-3-391. [DOI] [PubMed] [Google Scholar]
- Pearson A. D., Ellwood D. C. The effect of growth conditions on the chemical composition and endotoxicity of walls of Aerobacter aerogenes N.C.T.C. 418. Biochem J. 1972 Apr;127(3):72P–73P. doi: 10.1042/bj1270072pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Powell J. T., Fischlschweiger W., Birdsell D. C. Modification of surface composition of Actinomyces viscosus T14V and T14AV. Infect Immun. 1978 Dec;22(3):934–944. doi: 10.1128/iai.22.3.934-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson L. A., Little W., Hageage G. J. Application of fluorescent antibody methods in the analysis of plaque samples. J Dent Res. 1976 Jan;55:A80–A86. doi: 10.1177/002203457605500126011. [DOI] [PubMed] [Google Scholar]
- Van de Rijn I., Bleiweis A. S. Antigens of Streptococcus mutans. I. Characterization of a serotype-specific determinant from Streptococcus mutans. Infect Immun. 1973 May;7(5):795–804. doi: 10.1128/iai.7.5.795-804.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherell J. R., Jr, Bleiweis A. S. Antigens of Streptococcus mutans: characterization of a polysaccharide antigen from walls of strain GS-5. Infect Immun. 1975 Dec;12(6):1341–1348. doi: 10.1128/iai.12.6.1341-1348.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Woldringh C. L., Mirelman D. Constant peptidoglycan density in the sacculus of Escherichia coli B/r growing at different rates. FEBS Lett. 1979 Feb 1;98(1):29–32. doi: 10.1016/0014-5793(79)80144-1. [DOI] [PubMed] [Google Scholar]



