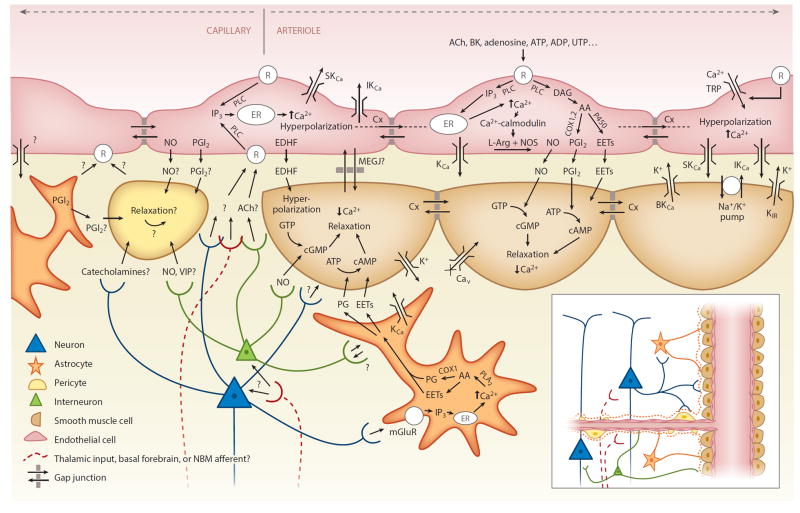
Figure 3.
Candidate neurovascular coupling pathways [modified from Félétou & Vanhoutte (2004) and Attwell et al. (2010)]. Astrocytes can sense glutamate via metabotropic glutamate receptors (mGluR) and increase their intracellular calcium (Ca2+), which can generate arachidonic acid (AA) from phospholipase A2 (PLA2) which is converted by COX1 (or 3) to prostaglandins (PG) and by P450 epoxygenase to epoxyeicosatrienoic acid (EETs). Both PGs and EETs can relax smooth muscle cells (SMCs) through conversion of adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). Endothelial cells can increase their intracellular calcium through transient receptor potential (TRP) cation channels, and in response to receptor (R) binding, through IP3-mediated release of calcium from intracellular stores [endoplasmic reticulum (ER)]. Endothelial receptor targets include acetylcholine (ACh), bradykinin (BK), adenosine diphosphate (ADP), ATP, uridine triphosphate (UTP), and adenosine. Receptor binding can activate phospholipase C (PLC) (or PLA2), which via diacyl-glycerol (DAG) can also produce EETs and AA derivatives including prostacyclin (PGI2), both of which can drive SMC relaxation via cAMP, while increased intracellular calcium can drive the production of endothelial nitric oxide (NO), which can affect SMC relaxation through conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP). Intracellular calcium increases also lead to endothelial hyperpolarization through opening of calcium-dependent potassium channels (KCa). Endothelial hyperpolarization could be coupled to adjacent SMCs through myoendothelial gap junctions (MEGJs) or some other endothelium-derived hyperpolarizing factor (EDHF) such as K+ efflux through endothelial SKCa and IKCa channels by activating KIR and/or the Na+/K+ ATPase. SMC hyperpolarization causes relaxation through inactivation of voltage-dependent calcium channels (Cav). Endothelial hyperpolarization can spread rapidly to adjacent endothelial cells, likely via gap junctions. Pericytes possess many SMC-like properties and could relax in response to NO and PGI2 from astrocytes, neurons, or endothelial cells or in response to neuropeptides such as vasointenstinal peptides (VIPs). Pericytes or astrocytes could also be involved in signaling to endothelial cells. Question marks represent many other potential signaling pathways yet to be identified. Additional abbreviation: NMB, nucleus basalis of Meynert.