Abstract
The treatment of musculoskeletal sarcomas has made vast strides in the last few decades. From an era where amputation was the only option to the current day function preserving resections and complex reconstructions has been a major advance. The objectives of extremity reconstruction after oncologic resection include providing skeletal stability where necessary, adequate wound coverage to allow early subsequent adjuvant therapy, optimising the aesthetic outcome and preservation of functional capability with early return to function. This article highlights the concepts of surgical margins in oncology, discusses the principles governing safe surgical resection in these tumors and summarises the current modalities and recent developments relevant to reconstruction after limb salvage. The rationale of choice of a particular resection modality, the unique challenges of reconstruction in skeletally immature individuals and the impact of adjuvant modalities like chemotherapy and radiotherapy on surgical outcomes are also discussed.
KEY WORDS: Bone tumor, reconstruction, sarcoma
INTRODUCTION
The last few decades have seen rapid strides in the field of musculoskeletal oncology. Function preserving alternatives have now become the norm without compromising on overall disease survival and have resulted in a documented improvement in overall quality of life of patients compared with those with an amputation.[1] The advent of better imaging modalities, more effective chemotherapy, improved radiotherapy techniques, a better understanding of anatomy with continuous refinement in surgical techniques and advances in prosthesis design and materials have all played a part in enabling this goal.
Though the number of limb salvage surgeries undertaken for malignant tumours of the extremity has increased the principles that govern surgical resection of bone and soft tissue tumours have remained unchanged. Limb salvage is recommended only if:
The ability to achieve adequate margins is not compromised. If the surgeon is unable to achieve adequate margins in his endeavour to salvage the limb then an amputation is preferred.
The salvaged limb will provide function superior to that offered by a prosthetic limb after an amputation. A desensate salvaged limb with inadequate motors defeats the very purpose of limb salvage, which aims at improving the patient's quality of life.
Balancing these two opposing goals can often be a Herculean challenge, especially in patients with large tumours. Kawaguchi's concept of “barrier effects” has helped surgeons better understand evaluation of margins of resection.[2] Though conventionally quantitative parameters were used to define resection margins Kawaguchi converted anatomical structures (any tissue that has resistance against tumour invasion like muscle fascia, joint capsule, tendon, tendon sheath, epineurium, vascular sheath, and joint cartilage) into definitive thickness of normal tissue and classified them as either a thick barrier or a thin barrier. For purposes of margin evaluation a thick barrier was equivalent of 3 cm thickness of normal tissue and a thin barrier was considered to be 2 cm. Intact joint cartilage was equivalent of 5 cm thickness of normal tissue. By considering barrier effects translated into concrete distance equivalents, oncologically safe surgery can be planned at sites where barriers exist by using margins less than those mandated by true physical distance.
Though it remains our endeavour to offer limb salvage to the majority of our patients certain adverse factors often make these surgeries more complex. Poorly placed biopsy incisions, major vascular involvement, encasement of a major motor nerve, preoperative infection and inadequate motors after resection are hurdles that may need to be overcome using the advances in microsurgical techniques that offer the ability to transfer motors, graft nerves and vessels and provide adequate soft tissue even after extensive resections of the overlying skin, muscles and neurovascular structures.[3]
Though surgical resection remains the mainstay of treatment in musculoskeletal tumours it is uncommon for a patient with a high grade sarcoma to be treated by surgery alone. Adjuvant modalities like chemotherapy and radiotherapy play an essential part in the integrated management of these patients and the surgeon must be aware that continuous interaction and coordination between the various treating disciplines is important in order to provide the different treatment modalities in the most optimum sequence at appropriate times. Surgery must be planned in such a manner so as not to unduly disrupt the delivery of these adjuvant modalities. Problems in wound healing can result in a delay in the postoperative delivery of these modalities, which could compromise both local and distant disease control. Both chemotherapy and radiotherapy can have a deleterious effect as far as surgery is concerned. Patients receiving chemotherapy are often immuno compromised resulting in an increased susceptibility to postoperative infection. Bone healing too may be delayed. Radiotherapy if administered preoperatively has been shown to increase the incidence of wound complications often necessitating the use of local or distant flaps primarily in an attempt to forestall these complications. Postoperative radiation too can result in delayed bone healing and union, radiation induced ulcers and wound dehiscence often exposing underlying bone, neurovascular structures and metallic prosthesis.[4] Postoperative rehabilitation and exercise regimes may need to be modified or personalised because of the use of tissue transfer at index surgery and are often disrupted and delayed due to the necessity of administering these adjuvant modalities.
Limb salvage therefore requires a well-coordinated multidisciplinary approach involving varied specialties. The objectives of extremity reconstruction after oncologic resection include providing skeletal stability where necessary, adequate wound coverage to allow early subsequent adjuvant therapy, optimising the aesthetic outcome and preservation of functional capability with early return to function.
This article summarises the current modalities and recent developments relevant to reconstruction after limb salvage.
BONE SARCOMAS
There are a variety of reconstruction options after excision of bone tumours. Metallic prostheses (megaprostheses), which span the resection gap and allow for movement of the joint form the mainstay in limb salvage surgery for reconstruction after tumour resection, providing both mobility and stability.[5] They provide an immediate return to function and unlike biologic alternatives (bone) are not affected by on-going adjuvant chemotherapy and radiotherapy. Low cost, locally manufactured prosthesis are now available and these remain the workhorse for surgeons in resource challenged settings for prosthetic reconstructions after limb salvage.[6,7] These prostheses are now routinely being used even for total bone resections and total femur and total humerus replacements are not uncommon[8,9] [Figure 1].
Figure 1.
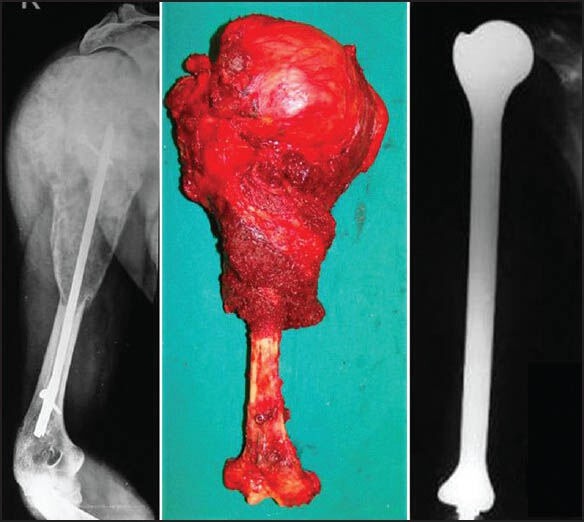
A case of malignant bone tumour of the humerus with an intramedullary implant in situ. Treated with wide excision and reconstructed with a total humerus prosthesis
A composite of an allograft (bone bank strut graft) and prosthesis can also be used for reconstruction in certain situations. An allograft replaces the segment of bone resected, while a prosthesis implanted in the allograft and host bone replaces the articular surface.[10] The allograft helps restore bone stock (which may be beneficial in a subsequent revision surgery) and provides a biological surface for soft tissue attachment, while the prosthesis provides a reliable and stable articulation and support for the allograft.
The advent of computer-assisted tumour surgery has increased the accuracy of intended bone resection and has also enabled multiplanar osteotomies.[11] These advances may be beneficial in resection and reconstruction of pelvic, sacral and difficult joint-preserving tumour surgery. They provide a useful tool in achieving a better balance between disease resection and preservation of function in anatomically challenging locations.[12] To further reduce technical errors during navigation-assisted bone tumour resection, surgeons have also experimented with direct magnetic resonance imaging (MRI)-guided navigation surgery without image fusion using absorbable pins as temporary implanted bone markers that prevent artifacts on MRI.[13] Computer aided design allows for the manufacture of customised prosthesis to accommodate for individual patient specific complex resections and reconstructions aimed at maximizing residual bone stock.[14]
Infection remains an inherent danger with the use of large metallic implants in immuno compromised patients.[15] Various agents coated on the external surface of the prosthesis have helped reduce the rate of infection. Silver coated prosthesis, vancomycin coated prosthesis and iodine impregnated implants have all shown promise in early results.[16,17,18]
Children, because of the dynamic nature of growing bones pose a unique challenge. The issue of ultimate limb length discrepancy at skeletal maturity also influences the choice of reconstruction, especially in the lower limb. The use of free vascularised epiphyseal transfer after resection of extremity tumours has not been commonly employed with only the occasional case reports present in the literature.[19,20] An expandable prosthesis is a commonly used solution to the problem of limb length discrepancy that would result in young children offered salvage with a megaprosthesis.[21] The newer generation expandable prostheses have special mechanisms to lengthen them at periodic intervals by allowing graduated extension when subjected to a controlled external magnetic field [Figure 2]. They thus permit noninvasive expansion on an outpatient basis obviating the need for repeated surgical exposures. However the high cost of these “expandable” prosthesis and limited availability of a “low cost” alternative precludes the use of this option in a large majority of growing children.
Figure 2.
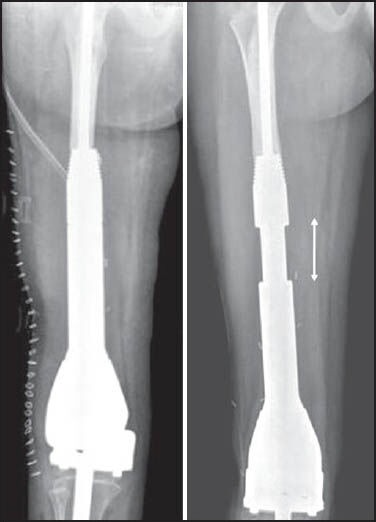
Immediate postoperative radiograph of an expandable prosthesis (left). The same prosthesis after serial expansion in vivo (right). The double headed arrow demonstrates the region of expansion
Where cost constraints preclude the use of expandable prosthesis in a majority of children with residual growth, rotationplasty remains an often used alternative.[22] Rotationplasty is also useful occasionally in adults where large tumours necessitate resection of extensive skin and surrounding soft tissue precluding conventional limb salvage. It is essentially an intercalary limb resection preserving the continuity of the neurovascular bundle. The most common site where rotationplasty is used is for tumours of the distal femur and proximal tibia. Patients undergo an en bloc excision of the distal femur and proximal tibia including skin and all surrounding soft tissues while maintaining the continuity of the neurovascular bundle. The bony limb continuity is then re-established by fusing the tibia with the proximal femoral remnant after 180° external limb rotation [Figure 3]. The vessel can either be dissected out or divided and reanastamosed but the sciatic nerve has to be carefully preserved and retained. Thus arguably, rotationplasty is an example of the largest “pedicled/free flap.” In case the vessels are not divided but dissected out, subsequent additional vessel length after limb shortening is accommodated for by carefully looping the vessels so as to avoid stasis. Limb rotation is necessary as otherwise the foot would end up pointing forwards. Once rotated however, the ankle now becomes the knee and the foot becomes a useful attachment for below-knee prosthesis. Ankle movements simulate knee movements and the patient has the equivalent of a functioning below-knee amputation rather than a high above knee one. A major advantage is that the sole being the normal weight bearing area there is no phantom pain. The stump can be left longer in children to account for subsequent growth of the contra lateral limb, so that the opposite knee and the repositioned rotated ankle of the operated limb would lie at the same level at skeletal maturity.
Figure 3.
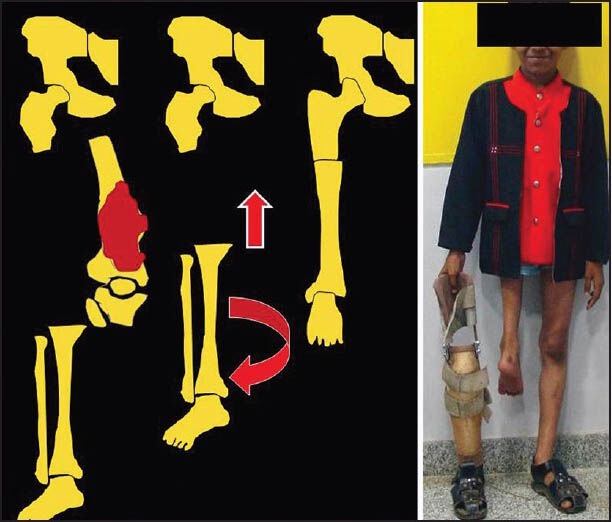
Pictorial representation of rotationplasty depicting excision of a lower end femur tumor followed by rotation and shortening of the distal limb. The image on the right shows the eventual clinical outcome with the prosthesis that is used
As skin, quadriceps and the vessel can be sacrificed to provide a wider margin this procedure is also applicable in cases with extensive involvement of the quadriceps or where skin has undergone prior radiation. It is also used to salvage cases with uncontrolled infection following a prosthetic replacement. For young children with tumours involving the entire femur it is possible to simply resect the femur and insert the lateral tibial plateau into the hip joint, producing a rotated limb with a false hip.[23] In young children this will remodel to produce a remarkably good hip joint. In older adults too, the same technique is applicable. Here instead of articulating the tibial plateau with the acetabulum, a cemented bipolar prosthesis is inserted into the proximal tibia, which in turn articulates with the acetabulum to form a “new hip joint.”
A 10-year follow-up study of patients with rotationplasty found no reduction in psychosocial adaptation, and similar life contentment as in healthy persons. Based on these findings, the authors recommended rotationplasty instead of amputation whenever conventional limb salvage was not possible.[24]
A similar concept utilising resection of all surrounding skin, soft tissue and bone to ensure adequate oncologic clearance is applicable in large or contaminated lesions of the upper limb around the elbow. The entire intercalary segment is resected leaving only neurovascular continuity between the distal and proximal fragments. Internal fixation between the residual humerus and ulna in the appropriate position facilitates a bony arthrodesis. Care must be taken to resect an adequate segment of the proximal radius to ensure that subsequent prono supination is retained. The resultant “fused elbow” retains excellent hand function. The upper limb though cosmetically shorter than the opposite side offers a residual function far better than after an above elbow amputation.
Biological means of reconstruction using autografts, allografts and re-implantation of sterilised tumour bone (after autoclaving/pasteurisation/irradiation) offer an attractive alternative option in certain scenarios. Conventional strut allografts and fibula autografts have the disadvantage of being unable to provide a mobile articulating surface. Thus spanning a defect involving the articular surface with these would result in an arthrodesis and inability to move that particular joint. Osteochondral allografts were used in an attempt to recreate a mobile joint but long term data has not been very encouraging.[25]
For tumours that involve the diaphyseal portion of a bone, an intercalary resection and reconstruction can be performed that saves the joints at either end. In these cases, the excised segment of bone can be replaced with either a metallic diaphyseal prosthesis or bone in the form of a strut allograft or fibular autograft. The pedicled or free vascularised fibula graft is among the most commonly used grafts in musculoskeletal oncology across various sites.[26,27] [Figure 4] While it was initially hoped that massive allografts would become fully incorporated into the host, retrieval data shows that only a small percentage of the allograft actually becomes revascularised, while the rest remains necrotic. Rather than a biologic replacement for the excised bone segment the allograft functions as a biologic spacer. Allografts have their share of complications too, which include infection, nonunion and late fractures.[28]
Figure 4.
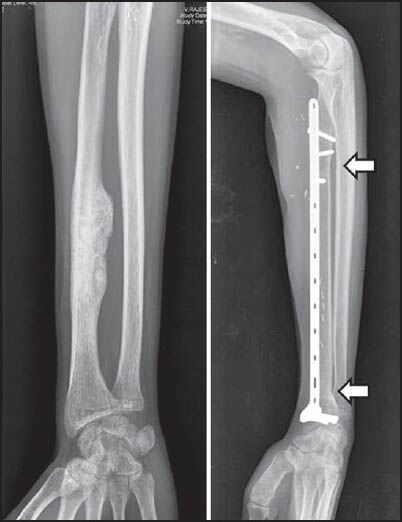
Preoperative and follow-up radiograph at 24 months of a diaphyseal osteosarcoma of the radius; excised and reconstructed with a vascularised fibula (arrows demonstrate the united osteotomy junctions)
A single vascularised fibula is often not strong enough to withstand the loading after reconstruction of large defects, especially in the lower extremity and fractures are not uncommon. In an attempt to improve the incorporation of allografts, while providing additional structural stability to the vascularised fibula, a combination using a strut allograft with a vascularised fibula autograft has been advocated[29,30] [Figures 5 and 6].
Figure 5.
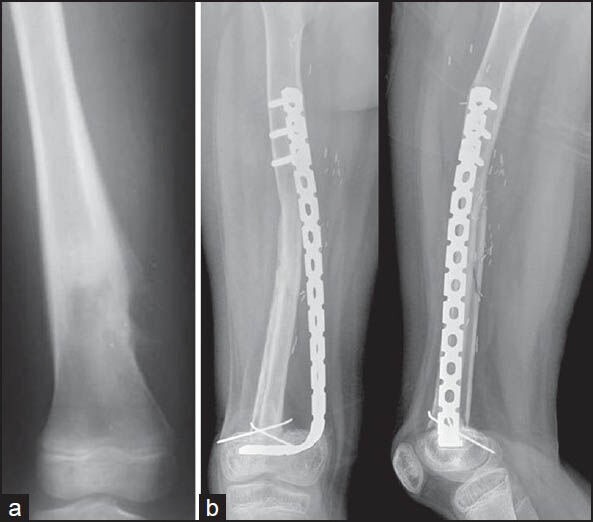
(a) Preoperative radiograph of a diaphyseal osteosarcoma of the femur, (b) follow-up radiograph at 24 months after excision and reconstruction with a combination of vascularised fibula and allograft
Figure 6.
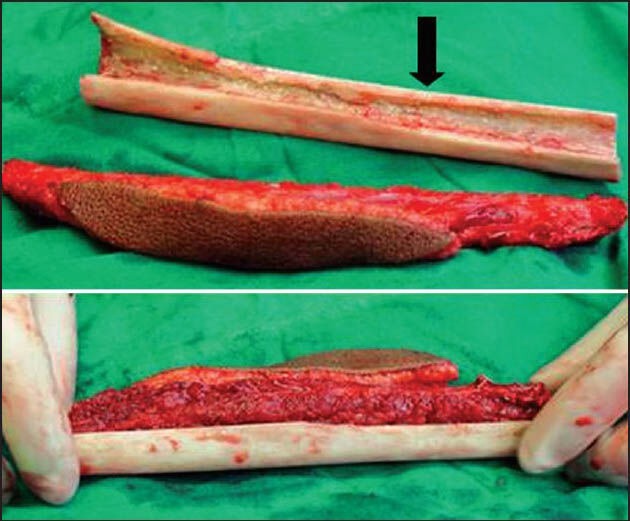
Combination of vascularised fibula and allograft (black arrow) used for reconstruction as shown in Figure 5
Sequential controlled bone transportation as proposed by Ilizarov has also been used to fill defects created after skeletal resection.[31] Defects are usually large and the process of bone regeneration can be a long drawn affair. The presence of multiple external pins and wires for extensive periods required by this method can be a problem in patients who are immuno compromised because of chemotherapy and therefore susceptible to pin tract infections. The quality of the bone regenerate may also be altered because of adjuvant therapies.
Recently there has been a lot of interest in using the patient's own tumour bone and replacing it after it has been sterilised.[32] Methods of sterilisation described have included the use of autoclaving, microwave, pasteurising, liquid nitrogen and radiotherapy (extracorporeal radiotherapy). The principle is the same; the tumour bearing bone is excised with adequate margins, all soft tissues and macroscopic tumour removed and the remaining bone sterilized by any of the above methods before being reimplanted. Although the bone is dead the advantage is that it functions as a “size matched” allograft. An essential prerequisite is that the bone should initially not be damaged significantly by the tumour otherwise it would become too weak to use once sterilised. The problems inherent with allograft usage remain and hence it too can be combined with a vascularised graft. The technique is relatively time consuming but inexpensive to use. As the patient's own bone is used it avoids the logistic issues involved in allograft procurement and the fear of disease transmission.
In the leg and the forearm the use of the “fellow bone” as a vascularised graft (without micro vascular anastamosis) is useful in reconstructing long segmental defects after resection. Fibular centralization or the “fibula-pro-tibia” procedure has been used in combination with strut allografts and resterlised tumour bone. It has also been described as a standalone technique for reconstructing these defects.[33] Following adequate resection of the tibia, the fibula is osteotomised proximally and distally at the appropriate level. It is then transposed medially keeping the entire soft tissue attachments intact and fixed proximally to the residual tibia and distally to the residual tibia or talus (in case of an intra-articular resection). This now functions as a vascularised graft resulting in rapid union with subsequent hypertrophy when subject to loading [Figure 7]. Ipsilateral fibula transfer is an easy technique that does not require micro vascular skills and can be accomplished by the primary operating surgeon himself. It also helps reduce operative time compared to transfers requiring vascular anastamosis. Volume reduction of the lower leg due to antero-medial shift of the fibula facilitates skin closure after tumour excision making this an attractive option in large tumours or in cases where excision of biopsy scar entails loss of excessive soft tissue.
Figure 7.
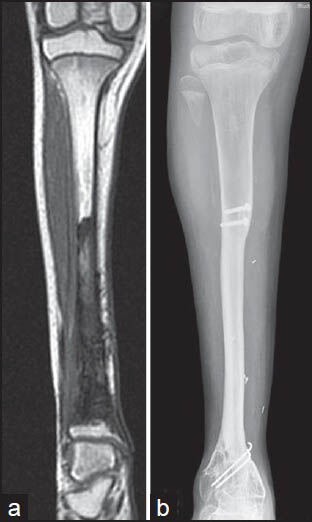
(a) Preoperative magnetic resonance imaging of a Ewing sarcoma of the tibia, (b) follow-up radiograph at 36 months showing hypertrophy of the transposed (medial translation into post excision defect) fibula
A similar concept is applicable in the upper limb. The distal ulna can be transposed to fill the defect left after resection of tumours of the distal radius and the wrist is arthrodesed.[34] As in the lower limb, volume reduction of the forearm due to radial shift of the ulna facilitates skin closure after tumour excision, especially in cases where fungating lesions or extensive soft tissue tumour components entail loss of soft tissue and skin. An ancillary benefit of the resultant one bone forearm is that the complications of ulnar variance which result when there is inappropriate restoration of length after use of a fibula, iliac crest graft or allograft can be avoided. In spite of creating a one bone forearm, an advantage of this technique is that it retains prono supination unlike when the ulna is directly fused to the carpus with centralisation of the carpus on the ulna.
The “induced membrane technique” has been described as an alternative for immediate biological reconstruction after tumour resection in children.[35] During the first stage, a cement spacer is inserted after bone resection and stabilisation. The cement spacer is removed during a second stage procedure performed after chemotherapy is completed and cortico-cancellous bone autograft is placed in the biological induced chamber created by the cement spacer. This bone grafts consolidate leading to rapid bone union. The advantage of this two stage procedure is that it helps reduce the operating time during the first stage and also reduces early complications despite major bone resection in patients receiving chemotherapy.
Improvement in function after resection is not restricted to the realm of limb salvage alone. Even in amputations the surgeon should endeavour to improve ultimate function by ensuring that optimal residual limb length is preserved or regained. When amputation just below the knee is necessary the remaining proximal tibia may be too short for a below-knee prosthesis, although the knee may be normal. Including the distal tibia or foot in a long posterior flap by turning it up and increasing the length of a very short proximal tibial stump is one of the methods described.[36] The knee is thereby saved, allowing satisfactory use of below-knee prosthesis. This technique is particularly applicable when the distal leg is normal and well vascularised. Even after hip disarticulation functional outcome can be improved by preserving a musculocutaneous flap and placing a modular endoprosthesis in the acetabulum.[37] It is worthwhile considering the use of autografts or allografts to augment residual limb length in patients for whom traditional amputation techniques would result in poor function, difficulty in fitting a prosthesis, or greater than necessary anatomic loss.[38]
Forequarter and hindquarter amputations are not uncommon for large proximal limb tumours. For these large defects that require a free flap, the distal uninvolved portions of these limbs can be harvested as fillet flaps and represent the “spare parts” concept of surgical reconstruction.[39] The use of the fillet flap has been shown to be oncologically sound, has no associated donor sites and permits rapid wound healing with an improvement in the quality of life.
The transcutaneous intraosseous prostheses though in an early stage have also demonstrated considerable success.[40] This new method which emphasises the direct attachment of skin, subcutaneous tissues and muscle to the prosthesis claims to decrease the problems associated with fixation of an exoprosthesis to a stump such as sweating, rubbing and difficulty in donning and doffing the prosthesis.
SOFT TISSUE SARCOMAS
Though surgical excision remains the mainstay of treatment, a vast majority of patients of soft tissue sarcoma would require radiotherapy as a component of limb salvage.[41] Radiotherapy may be delivered either as pre or postoperative radiotherapy. Split skin grafts fare poorly when subjected to radiation and hence it is preferable to have a robust flap for soft tissue cover in case primary closure is not possible following resection. Incisions closed under tension are also liable to break down during or after radiotherapy leading to prolonged delays in wound healing with a detrimental cascading effect on the delivery of adjuvant treatment modalities. In case the underlying bone or metallic prosthesis is exposed, a flap is mandatory in order to cover it and enable early wound healing and rehabilitation. Thus a relaxed, tension free, robust soft tissue cover is the cornerstone to ensure that heroic resections are not doomed to disaster in a milieu that is otherwise detrimental to wound healing. Patients with tumours of the lower extremity involving major neurovascular structures and for whom radiation therapy is planned have an increased risk of a nonhealing wound following resection which may necessitate a delayed local or free vascularised tissue transfer.[42] It has been shown that there is a tendency of higher wound complication rates in patients who are referred for late reconstruction and the early involvement of plastic surgeons can help reduce this problem.[43]
A recent report suggests that acellular dermis reconstruction offers an excellent coverage alternative after excision of cutaneous and soft tissue malignancies in patients with limited options of native tissue coverage.[44] It serves as a bridge to permanent reconstruction or as a permanent biologic dressing of complex surgical defects. Even in situations in which adjuvant radiation was needed, AlloDerm was used without major complications.
Of late, there has been growing interest in the use of the vacuum-assisted closure (VAC) device (a form of negative pressure wound therapy) to promote wound healing.[45,46] VAC has been shown to be safe and effective and is associated with lower overall complications rates, infection rate, and the need for further surgery, while managing wound healing in sarcoma patients following surgery. It can also be used to prepare the wound bed for grafting in high-risk patients who are not candidates for more complex reconstructions.[45]
Flaps when used can be local or distant, pedicled or free, depending on the anatomic area affected and the condition of the surrounding tissues whether it has been violated by prior surgery or not [Figure 8]. Innervated free or pedicled musculocutaneous flap transfers for reconstruction after resection involving the extremities help retain the muscle strength of the reconstructed compartment and restore the range of motion.[47,48] In an attempt to decrease donor-site morbidity surgeons have successfully used the assistance of the robotic da Vinci Surgical System to harvest the rectus abdominis muscle for free tissue transfer to a lower extremity defect.[49] Supercharging large flaps to augment their blood supply and increase their reliability is also an often used alternative when large tissue bulk is harvested for coverage of massive defects.[50,51]
Figure 8.
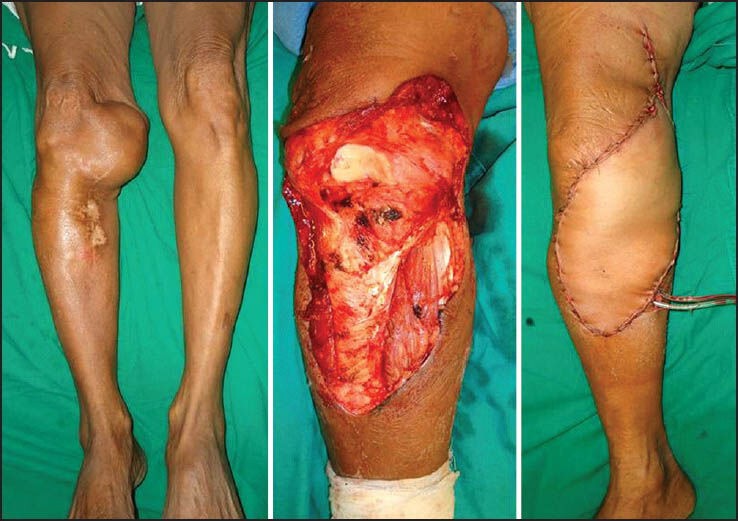
Recurrent synovial sarcoma of the leg. Treated with wide excision and the defect covered with a free antero lat thigh flap
It is preferable to start raising flaps only after primary resection is complete. An intraoperative frozen section may reveal compromised margins often mandating the removal of additional tissue. Thus if the flap has been harvested simultaneously during the excision it may occasionally fall short after the revised excision. Sometimes, the adjacent/regional tissue may not be suitable for transfer because its vascular pedicle may have been ligated during tumour resection or damaged by prior radiotherapy thus necessitating a microvascular tissue transfer. Recent studies comparing the outcomes of free tissue transfer in preoperatively irradiated patients compared with nonirradiated patients conclude that free tissue transfer is safe and effective in sarcoma patients undergoing surgical resection and reconstruction following neoadjuvant radiotherapy and is associated with fewer late recipient site complications than after postoperative irradiation.[52,53]
Occasionally the plastic surgeon may be called on to transfer muscles or tendons when certain motor groups have been resected in an attempt to achieve adequate margins. Sarcomas as a rule tend to displace vessels and nerves and do not infiltrate them primarily. If the nerve and/or vessel is encased by a large tumour or infiltrated in the event of prior surgery, resection of the involved vessel or nerve would require appropriate reconstruction.[54] Vascular replacement can be achieved using interpositional vein grafts or alloplastic material.[55] If necessary, flow through flaps can be used to reconstruct both, the vascular defects and the defects in bone and soft tissue.[56,57]
CONCLUSION
The surgeon must decide with the patient what the best surgical procedure is for that individual and he is then responsible for achieving adequate margins and reconstructing the limb if limb salvage is chosen. Properly indicated and executed limb salvage offers the advantage of better function and psychological benefits resulting in an overall improvement in quality of life. It entails a well-orchestrated effort involving various specialties and better outcomes are likely to be achieved with centralisation of expertise at regional centres so that surgeons and their teams can offer a full range of surgical options to their patients, based upon experience and knowledge. The cost of treatment can be expensive and the postoperative rehabilitation is prolonged often requiring increased inpatient hospital care. Striking the right balance between adequate resection, while yet retaining or reconstructing tissue for acceptable function and cosmesis is a difficult task and complications are not uncommon.[58] Patients and their families need to be counselled regarding the potential setbacks that may occur in the course of their road to recovery, but the eventual satisfaction achieved by both, patient and surgeon after a successful limb salvage is unparalleled and is the elixir that drives oncology surgeons to sail into uncharted waters and to scale new heights.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Mason GE, Aung L, Gall S, Meyers PA, Butler R, Krüg S, et al. Quality of life following amputation or limb preservation in patients with lower extremity bone sarcoma. Front Oncol. 2013;3:210. doi: 10.3389/fonc.2013.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kawaguchi N, Ahmed AR, Matsumoto S, Manabe J, Matsushita Y. The concept of curative margin in surgery for bone and soft tissue sarcoma. Clin Orthop Relat Res. 2004;419:165–72. doi: 10.1097/00003086-200402000-00027. [DOI] [PubMed] [Google Scholar]
- 3.Pollock RC, Stalley PD. Biopsy of musculoskeletal tumours — beware. ANZ J Surg. 2004;74:516–9. doi: 10.1111/j.1445-2197.2004.03060.x. [DOI] [PubMed] [Google Scholar]
- 4.Jeys LM, Luscombe JS, Grimer RJ, Abudu A, Tillman RM, Carter SR. The risks and benefits of radiotherapy with massive endoprosthetic replacement. J Bone Joint Surg Br. 2007;89:1352–5. doi: 10.1302/0301-620X.89B10.19233. [DOI] [PubMed] [Google Scholar]
- 5.Gosheger G, Gebert C, Ahrens H, Streitbuerger A, Winkelmann W, Hardes J. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin Orthop Relat Res. 2006;450:164–71. doi: 10.1097/01.blo.0000223978.36831.39. [DOI] [PubMed] [Google Scholar]
- 6.Tang X, Guo W, Yang R, Tang S, Yang Y. Custom-made prosthesis replacement for reconstruction of elbow after tumor resection. J Shoulder Elbow Surg. 2009;18:796–803. doi: 10.1016/j.jse.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Natarajan MV, Sivaseelam A, Ayyappan S, Bose JC, Sampath Kumar M. Distal femoral tumours treated by resection and custom mega-prosthetic replacement. Int Orthop. 2005;29:309–13. doi: 10.1007/s00264-005-0677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puri A, Gulia A. The results of total humeral replacement following excision for primary bone tumour. J Bone Joint Surg Br. 2012;94:1277–81. doi: 10.1302/0301-620X.94B9.29697. [DOI] [PubMed] [Google Scholar]
- 9.Puri A, Gulia A, Chan WH. Functional and oncologic outcomes after excision of the total femur in primary bone tumors: Results with a low cost total femur prosthesis. Indian J Orthop. 2012;46:470–4. doi: 10.4103/0019-5413.98834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donati D, Colangeli M, Colangeli S, Di Bella C, Mercuri M. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin Orthop Relat Res. 2008;466:459–65. doi: 10.1007/s11999-007-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Wang Z, Guo Z, Chen GJ, Yang M, Pei GX. Irregular osteotomy in limb salvage for juxta-articular osteosarcoma under computer-assisted navigation. J Surg Oncol. 2012;106:411–6. doi: 10.1002/jso.23105. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Wang Z, Guo Z, Chen GJ, Yang M, Pei GX. Precise resection and biological reconstruction under navigation guidance for young patients with juxta-articular bone sarcoma in lower extremity: Preliminary report. J Pediatr Orthop. 2014;34:101–8. doi: 10.1097/BPO.0b013e31829b2f23. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Kang HG, Kim HS. MRI-guided navigation surgery with temporary implantable bone markers in limb salvage for sarcoma. Clin Orthop Relat Res. 2010;468:2211–7. doi: 10.1007/s11999-009-1209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong KC, Kumta SM, Sze KY, Wong CM. Use of a patient-specific CAD/CAM surgical jig in extremity bone tumor resection and custom prosthetic reconstruction. Comput Aided Surg. 2012;17:284–93. doi: 10.3109/10929088.2012.725771. [DOI] [PubMed] [Google Scholar]
- 15.Pilge H, Gradl G, von Eisenhart-Rothe R, Gollwitzer H. Incidence and outcome after infection of megaprostheses. Hip Int. 2012;22(Suppl 8):S83–90. doi: 10.5301/HIP.2012.9576. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya H, Shirai T, Nishida H, Murakami H, Kabata T, Yamamoto N, et al. Innovative antimicrobial coating of titanium implants with iodine. J Orthop Sci. 2012;17:595–604. doi: 10.1007/s00776-012-0247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussmann B, Johann I, Kauther MD, Landgraeber S, Jäger M, Lendemans S. Measurement of the silver ion concentration in wound fluids after implantation of silver-coated megaprostheses: Correlation with the clinical outcome. Biomed Res Int 2013. 2013:763096. doi: 10.1155/2013/763096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101:389–95. doi: 10.1002/jso.21498. [DOI] [PubMed] [Google Scholar]
- 19.Erdmann D, Garcia RM, Blueschke G, Brigman BE, Levin LS. Vascularized fibula-based physis transfer for pediatric proximal humerus reconstruction. Plast Reconstr Surg. 2013;132:281e–87. doi: 10.1097/PRS.0b013e31829589fb. [DOI] [PubMed] [Google Scholar]
- 20.Fang B, Yi C, Zhang H, Zhang Q, Li Y, Wei Q, et al. Combined epiphyseal preservation and autograft bone transfer in treatment of children osteosarcoma. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2013;27:45–9. [PubMed] [Google Scholar]
- 21.Ruggieri P, Mavrogenis AF, Pala E, Romantini M, Manfrini M, Mercuri M. Outcome of expandable prostheses in children. J Pediatr Orthop. 2013;33:244–53. doi: 10.1097/BPO.0b013e318286c178. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal M, Puri A, Anchan C, Shah M, Jambhekar N. Rotationplasty for bone tumors: Is there still a role? Clin Orthop Relat Res. 2007;459:76–81. doi: 10.1097/BLO.0b013e31805470f0. [DOI] [PubMed] [Google Scholar]
- 23.Winkelmann WW. Type-B-IIIa hip rotationplasty: An alternative operation for the treatment of malignant tumors of the femur in early childhood. J Bone Joint Surg Am. 2000;82:814–28. doi: 10.2106/00004623-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Rödl RW, Pohlmann U, Gosheger G, Lindner NJ, Winkelmann W. Rotationplasty — quality of life after 10 years in 22 patients. Acta Orthop Scand. 2002;73:85–8. doi: 10.1080/000164702317281468. [DOI] [PubMed] [Google Scholar]
- 25.Ogilvie CM, Crawford EA, Hosalkar HS, King JJ, Lackman RD. Long-term results for limb salvage with osteoarticular allograft reconstruction. Clin Orthop Relat Res. 2009;467:2685–90. doi: 10.1007/s11999-009-0726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bach AD, Kopp J, Stark GB, Horch RE. The versatility of the free osteocutaneous fibula flap in the reconstruction of extremities after sarcoma resection. World J Surg Oncol. 2004;2:22. doi: 10.1186/1477-7819-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beris AE, Lykissas MG, Korompilias AV, Vekris MD, Mitsionis GI, Malizos KN, et al. Vascularized fibula transfer for lower limb reconstruction. Microsurgery. 2011;31:205–11. doi: 10.1002/micr.20841. [DOI] [PubMed] [Google Scholar]
- 28.Dick HM, Strauch RJ. Infection of massive bone allografts. Clin Orthop Relat Res. 1994;306:46–53. [PubMed] [Google Scholar]
- 29.Abed YY, Beltrami G, Campanacci DA, Innocenti M, Scoccianti G, Capanna R. Biological reconstruction after resection of bone tumours around the knee: Long-term follow-up. J Bone Joint Surg Br. 2009;91:1366–72. doi: 10.1302/0301-620X.91B10.22212. [DOI] [PubMed] [Google Scholar]
- 30.Innocenti M, Abed YY, Beltrami G, Delcroix L, Manfrini M, Capanna R. Biological reconstruction after resection of bone tumors of the proximal tibia using allograft shell and intramedullary free vascularized fibular graft: Long-term results. Microsurgery. 2009;29:361–72. doi: 10.1002/micr.20668. [DOI] [PubMed] [Google Scholar]
- 31.Erler K, Yildiz C, Baykal B, Atesalp AS, Ozdemir MT, Basbozkurt M. Reconstruction of defects following bone tumor resections by distraction osteogenesis. Arch Orthop Trauma Surg. 2005;125:177–83. doi: 10.1007/s00402-005-0795-5. [DOI] [PubMed] [Google Scholar]
- 32.Puri A, Gulia A, Jambhekar N, Laskar S. The outcome of the treatment of diaphyseal primary bone sarcoma by resection, irradiation and re-implantation of the host bone: Extracorporeal irradiation as an option for reconstruction in diaphyseal bone sarcomas. J Bone Joint Surg Br. 2012;94:982–8. doi: 10.1302/0301-620X.94B7.28916. [DOI] [PubMed] [Google Scholar]
- 33.Puri A, Subin BS, Agarwal MG. Fibular centralisation for the reconstruction of defects of the tibial diaphysis and distal metaphysis after excision of bone tumours. J Bone Joint Surg Br. 2009;91:234–9. doi: 10.1302/0301-620X.91B2.21272. [DOI] [PubMed] [Google Scholar]
- 34.Puri A, Gulia A, Agarwal MG, Reddy K. Ulnar translocation after excision of a Campanacci grade-3 giant-cell tumour of the distal radius: An effective method of reconstruction. J Bone Joint Surg Br. 2010;92:875–9. doi: 10.1302/0301-620X.92B6.23194. [DOI] [PubMed] [Google Scholar]
- 35.Chotel F, Nguiabanda L, Braillon P, Kohler R, Bérard J, Abelin-Genevois K. Induced membrane technique for reconstruction after bone tumor resection in children: A preliminary study. Orthop Traumatol Surg Res. 2012;98:301–8. doi: 10.1016/j.otsr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 36.Pant R, Younge D. Turn-up bone flap for lengthening the below-knee amputation stump. J Bone Joint Surg Br. 2003;85:171–3. doi: 10.1302/0301-620x.85b2.13252. [DOI] [PubMed] [Google Scholar]
- 37.Gosheger G, Hillmann A, Rödl R, Ozaki T, Gebert C, Winkelmann W. Stump lengthening after hip disarticulation using a modular endoprosthesis in 5 patients. Acta Orthop Scand. 2001;72:533–6. doi: 10.1080/000164701753532899. [DOI] [PubMed] [Google Scholar]
- 38.Mohler DG, Kessler JI, Earp BE. Augmented amputations of the lower extremity. Clin Orthop Relat Res. 2000;371:183–97. doi: 10.1097/00003086-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Ver Halen JP, Yu P, Skoracki RJ, Chang DW. Reconstruction of massive oncologic defects using free fillet flaps. Plast Reconstr Surg. 2010;125:913–22. doi: 10.1097/PRS.0b013e3181cb6548. [DOI] [PubMed] [Google Scholar]
- 40.Pendegrass CJ, Gordon D, Middleton CA, Sun SN, Blunn GW. Sealing the skin barrier around transcutaneous implants: In vitro study of keratinocyte proliferation and adhesion in response to surface modifications of titanium alloy. J Bone Joint Surg Br. 2008;90:114–21. doi: 10.1302/0301-620X.90B1.19580. [DOI] [PubMed] [Google Scholar]
- 41.Puri A, Gulia A. Management of extremity soft tissue sarcomas. Indian J Orthop. 2011;45:301–6. doi: 10.4103/0019-5413.82332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz A, Rebecca A, Smith A, Casey W, Ashman J, Gunderson L, et al. Risk factors for significant wound complications following wide resection of extremity soft tissue sarcomas. Clin Orthop Relat Res. 2013;471:3612–7. doi: 10.1007/s11999-013-3130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marré D, Buendía J, Hontanilla B. Complications following reconstruction of soft-tissue sarcoma: Importance of early participation of the plastic surgeon. Ann Plast Surg. 2012;69:73–8. doi: 10.1097/SAP.0b013e31821ee497. [DOI] [PubMed] [Google Scholar]
- 44.Deneve JL, Turaga KK, Marzban SS, Puleo CA, Sarnaik AA, Gonzalez RJ, et al. Single-institution outcome experience using AlloDerm ® as temporary coverage or definitive reconstruction for cutaneous and soft tissue malignancy defects. Am Surg. 2013;79:476–82. [PMC free article] [PubMed] [Google Scholar]
- 45.Senchenkov A, Petty PM, Knoetgen J, 3rd, Moran SL, Johnson CH, Clay RP. Outcomes of skin graft reconstructions with the use of Vacuum Assisted Closure (VAC(R)) dressing for irradiated extremity sarcoma defects. World J Surg Oncol. 2007;5:138. doi: 10.1186/1477-7819-5-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakellariou VI, Mavrogenis AF, Papagelopoulos PJ. Negative-pressure wound therapy for musculoskeletal tumor surgery. Adv Skin Wound Care. 2011;24:25–30. doi: 10.1097/01.ASW.0000392924.75970.b9. [DOI] [PubMed] [Google Scholar]
- 47.Muramatsu K, Ihara K, Doi K, Yoshida K, Iwanaga R, Hashimoto T, et al. Functional neuro-vascularized muscle transfer for oncological reconstruction of extremity sarcoma. Surg Oncol. 2012;21:263–8. doi: 10.1016/j.suronc.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Grinsell D, Di Bella C, Choong PF. Functional reconstruction of sarcoma defects utilising innervated free flaps. Sarcoma 2012. 2012:315190. doi: 10.1155/2012/315190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel NV, Pedersen JC. Robotic harvest of the rectus abdominis muscle: A preclinical investigation and case report. J Reconstr Microsurg. 2012;28:477–80. doi: 10.1055/s-0031-1287674. [DOI] [PubMed] [Google Scholar]
- 50.Komorowska-Timek E, Gurtner G, Lee GK. Supercharged reverse pedicle anterolateral thigh flap in reconstruction of a massive defect: A case report. Microsurgery. 2010;30:397–400. doi: 10.1002/micr.20761. [DOI] [PubMed] [Google Scholar]
- 51.Gordley K, Marco R, Klebuc M. Supercharged internal hemipelvectomy that enhances flap perfusion and decreases wound-healing complications. Orthopedics. 2008;31:12. doi: 10.3928/01477447-20081201-09. [DOI] [PubMed] [Google Scholar]
- 52.Townley WA, Mah E, O’Neill AC, Wunder JS, Ferguson PC, Zhong T, et al. Reconstruction of sarcoma defects following pre-operative radiation: Free tissue transfer is safe and reliable. J Plast Reconstr Aesthet Surg. 2013;66:1575–9. doi: 10.1016/j.bjps.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 53.Chao AH, Chang DW, Shuaib SW, Hanasono MM. The effect of neoadjuvant versus adjuvant irradiation on microvascular free flap reconstruction in sarcoma patients. Plast Reconstr Surg. 2012;129:675–82. doi: 10.1097/PRS.0b013e3182412a39. [DOI] [PubMed] [Google Scholar]
- 54.Ghert MA, Davis AM, Griffin AM, Alyami AH, White L, Kandel RA, et al. The surgical and functional outcome of limb-salvage surgery with vascular reconstruction for soft tissue sarcoma of the extremity. Ann Surg Oncol. 2005;12:1102–10. doi: 10.1245/ASO.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 55.Muramatsu K, Ihara K, Miyoshi T, Yoshida K, Taguchi T. Clinical outcome of limb-salvage surgery after wide resection of sarcoma and femoral vessel reconstruction. Ann Vasc Surg. 2011;25:1070–7. doi: 10.1016/j.avsg.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Deune EG, Rodriguez E, Hatef D, Frassica F. Arterialized venous flow-through flap for simultaneous reconstruction of a radial artery defect and palmar forearm soft-tissue loss from sarcoma resection. J Reconstr Microsurg. 2005;21:85–91. doi: 10.1055/s-2005-864840. [DOI] [PubMed] [Google Scholar]
- 57.Garvey PB, Clemens MW, Rhines LD, Sacks JM. Vertical rectus abdominis musculocutaneous flow-through flap to a free fibula flap for total sacrectomy reconstruction. Microsurgery. 2013;33:32–8. doi: 10.1002/micr.21990. [DOI] [PubMed] [Google Scholar]
- 58.Renard AJ, Veth RP, Schreuder HW, van Loon CJ, Koops HS, van Horn JR. Function and complications after ablative and limb-salvage therapy in lower extremity sarcoma of bone. J Surg Oncol. 2000;73:198–205. doi: 10.1002/(sici)1096-9098(200004)73:4<198::aid-jso3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]


