Abstract
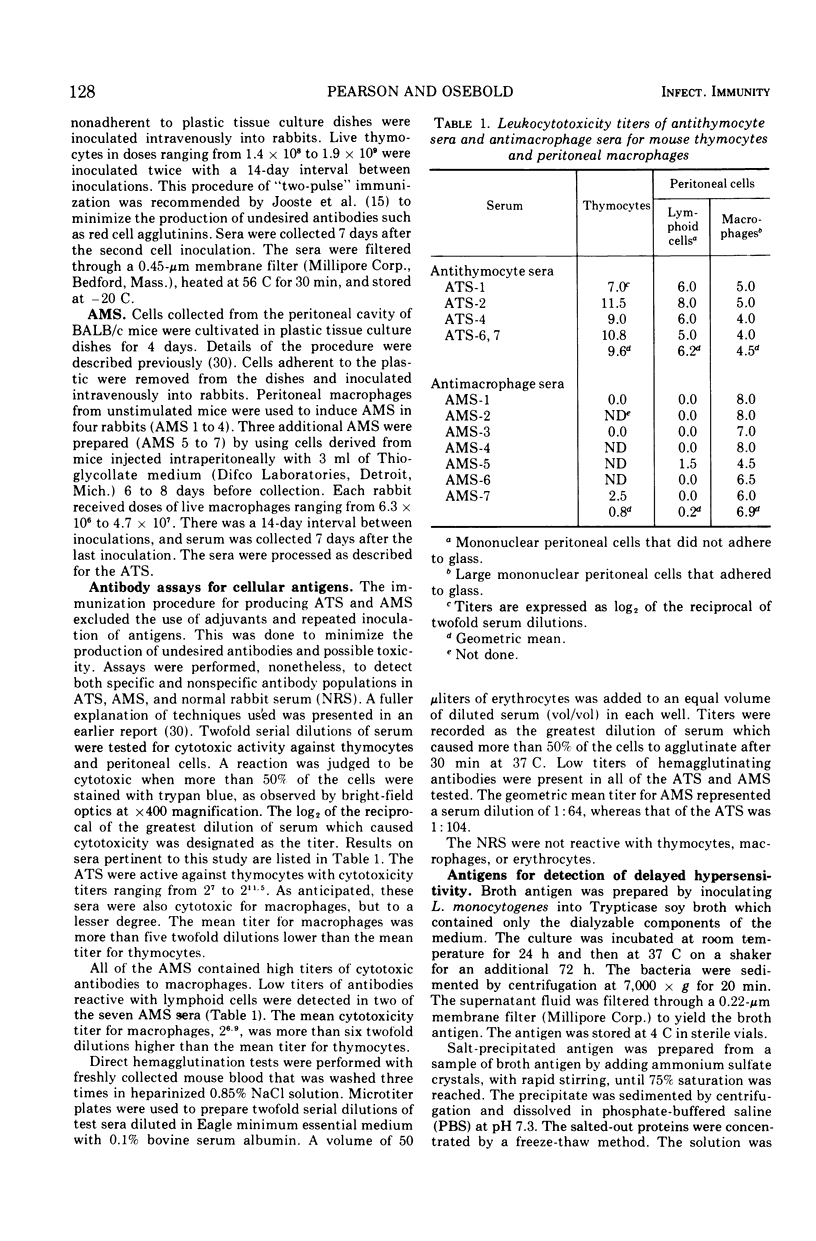
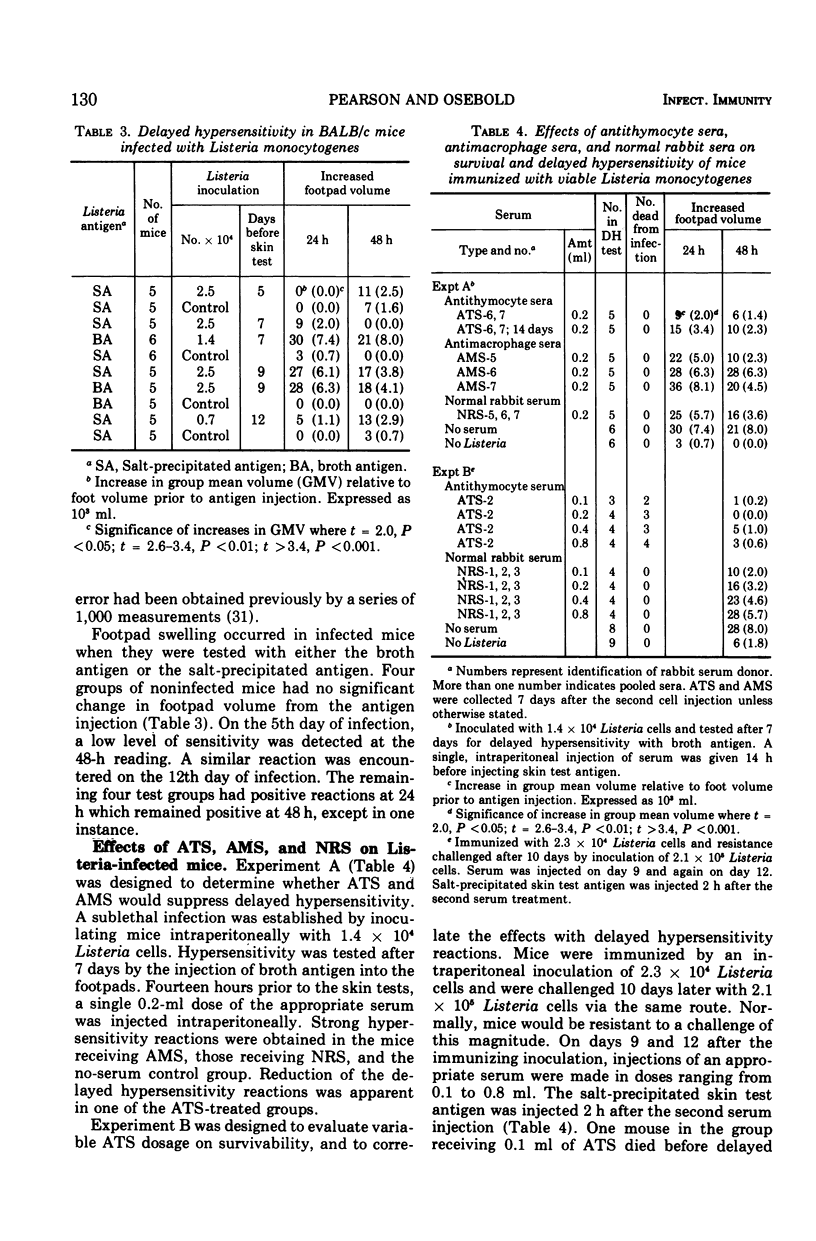
The cell-mediated immune responses of allograft rejection, delayed hypersensitivity, and resistance to Listeria monocytogenes were suppressed by injections of antithymocyte serum (ATS), but the immune responses were not significantly altered by antimacrophage serum (AMS) or normal rabbit serum (NRS). Antisera were prepared in rabbits against purified mouse thymocytes and purified peritoneal macrophages. When mice were injected with ATS near the time of skin grafting, allografts survived significantly longer. Similar administration of AMS or NRS failed to alter the course of graft rejection. Decreased footpad swelling indicated the suppression of delayed hypersensitivity in mice injected with ATS 6 days after a sublethal inoculation of Listeria cells. None of the serum treatments affected the ultimate survival of mice infected with a small number of bacteria. Either ATS or NRS was injected into immunized mice 1 day before and 2 days after a challenge inoculation of Listeria cells. Pronounced suppression of delayed hypersensitivity was found in the ATS-treated groups, along with extensive mortalities that reached 100% in the group receiving the largest dose of ATS. All control animals survived and demonstrated strong delayed hypersensitivity reactions. Antimacrophage serum had no significant effect on the three mechanisms of cell-mediated immunity that were tested. The lymphoid cells which mediate delayed hypersensitivity, antimicrobial cellular immunity, and allograft rejection possess antigenic determinants in common with thymocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson D. M., Cozad G. C. Effect of antilymphocyte serum on animals experimentally infected with Histoplasma capsulatum or Cryptococcus neoformans. J Bacteriol. 1969 Dec;100(3):1271–1276. doi: 10.1128/jb.100.3.1271-1276.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY D. W., USAMA B. A rapid method of grafting skin on tails of mice. Transplant Bull. 1960 Apr;7:424–425. doi: 10.1097/00006534-196004000-00045. [DOI] [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Quantitative studies on tissue transplantation immunity. II. The origin, strength and duration of actively and adoptively acquired immunity. Proc R Soc Lond B Biol Sci. 1954 Dec 15;143(910):58–80. doi: 10.1098/rspb.1954.0054. [DOI] [PubMed] [Google Scholar]
- Benacerraf B., Green I. Cellular hypersensitivity. Annu Rev Med. 1969;20:141–154. doi: 10.1146/annurev.me.20.020169.001041. [DOI] [PubMed] [Google Scholar]
- Blanden R. V., Langman R. E. Cell-mediated immunity to bacterial infection in the mouse. Thymus-derived cells as effectors of acquired resistance to Listeria monocytogenes. Scand J Immunol. 1972;1(4):379–391. doi: 10.1111/j.1365-3083.1972.tb03304.x. [DOI] [PubMed] [Google Scholar]
- Blanden R. V. Mechanisms of recovery from a generalized viral infection: mousepox. I. The effects of anti-thymocyte serum. J Exp Med. 1970 Nov;132(5):1035–1054. doi: 10.1084/jem.132.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman C., Feldman J. D. Composition, morphology, and source of cells in delayed skin reactions. Am J Pathol. 1970 Feb;58(2):201–218. [PMC free article] [PubMed] [Google Scholar]
- CROWLE A. J. Delayed hypersensitivity in several strains of mice studied with six different tests. J Allergy. 1959 Sep-Oct;30:442–459. doi: 10.1016/0021-8707(59)90023-1. [DOI] [PubMed] [Google Scholar]
- Dodd R. Y., Nash C. Modification of Listeria monocytogenes infection during unrelated cell-mediated immune reactions. J Reticuloendothel Soc. 1971 Dec;10(6):501–514. [PubMed] [Google Scholar]
- Dyminski J. W., Argyris B. F. Prolongation of allograft survival with antimacrophage serum. Transplantation. 1969 Nov;8(5):595–601. doi: 10.1097/00007890-196911000-00006. [DOI] [PubMed] [Google Scholar]
- Feldman J. D., Unanue E. R. Role of macrophages in delayed hypersensitivity. II. Effects of anti-macrophage antibody. Cell Immunol. 1971 Jun;2(3):275–282. doi: 10.1016/0008-8749(71)90047-5. [DOI] [PubMed] [Google Scholar]
- Goihman-Yahr M. Raffel S, Ferraresi RW: Delayed hypersensitivity in relation to suppression of growth of Listeria monocytogenes by guinea pig macrophages. J Bacteriol. 1969 Nov;100(2):635–640. doi: 10.1128/jb.100.2.635-640.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise E. R., Weiser R. S. Tuberculin sensitivity: the effect of antilymphocyte and antimacrophage serum on cutaneous, systemic and in vitro reactions. J Immunol. 1970 Mar;104(3):704–709. [PubMed] [Google Scholar]
- James K. Anti-lymphocyte serum. Clin Chim Acta. 1968 Sep;22(1):101–113. doi: 10.1016/0009-8981(68)90254-4. [DOI] [PubMed] [Google Scholar]
- Jooste S. V., Lance E. M., Levey R. H., Medawar P. B., Ruszkiewicz M., Sharman R., Taub R. N. Notes on the preparation and assay of anti-lymphocytic serum for use in mice. Immunology. 1968 Nov;15(5):697–705. [PMC free article] [PubMed] [Google Scholar]
- LITCHFIELD J. T., Jr A method for rapid graphic solution of time-per cent effect curves. J Pharmacol Exp Ther. 1949 Dec;97(4):399-408, 3 tab. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewi G., Temple A., Nind A. P., Axelrad M. A study of the effects of anti-macrophage sera. Immunology. 1969 Jan;16(1):99–106. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLUSKEY R. T., BENACERRAF B., MCCLUSKEY J. W. STUDIES ON THE SPECIFICITY OF THE CELLULAR INFILTRATE IN DELAYED HYPERSENSITIVITY REACTIONS. J Immunol. 1963 Mar;90:466–477. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Mackaness G. B., Hill W. C. The effect of anti-lymphocyte globulin on cell-mediated reistance to infection. J Exp Med. 1969 May 1;129(5):993–1012. doi: 10.1084/jem.129.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- Mandel M. A., DeCosse J. J. The effects of heterologous antithymocyte sera in mice. IV. Alteration of the growth rate of plasma cell tumors. J Immunol. 1969 Dec;103(6):1288–1293. [PubMed] [Google Scholar]
- Marshall V. R., Knight P. R. The effect of antilymphocytic serum (ALS) on the reticuloendothelial system (RES) of mice. J Immunol. 1969 Jun;102(6):1498–1503. [PubMed] [Google Scholar]
- Monaco A. P., Wood M. L., Gray J. G., Russell P. S. Studies on heterologous anti-lymphocyte serum in mice. II. Effect on the immune response. J Immunol. 1966 Feb;96(2):229–238. [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. D., Osebold J. W. Effects of antithymocyte and antimacrophage sera on the survival of mice infected with Listeria monocytogenes. Infect Immun. 1973 Mar;7(3):479–486. doi: 10.1128/iai.7.3.479-486.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson L. D., Osebold J. W., Wagner P. C. A device for measuring the volume of footpad swelling from delayed hypersensitivity reactions in mice. Lab Anim Sci. 1971 Aug;21(4):591–593. [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Youmans G. P. The role of lymphocytes and other factors in antimicrobial cellular immunity. J Reticuloendothel Soc. 1971 Jul;10(1):100–119. [PubMed] [Google Scholar]
- Zisman B., Hirsch M. S., Allison A. C. Selective effects of anti-macrophage serum, silica and anti-lymphocyte serum on pathogenesis of herpes virus infection of young adult mice. J Immunol. 1970 May;104(5):1155–1159. [PubMed] [Google Scholar]


