Abstract
There are numerous available biodegradable materials that can be used as scaffolds in regenerative medicine. Currently, there is a huge emphasis on the designing phase of the scaffolds. Materials can be designed to have different properties in order to match the specific application. Modifying scaffolds enhances their bioactivity and improves the regeneration capacity. Modifications of the scaffolds can be later characterized using several tissue engineering tools. In addition to the material, cell source is an important component of the regeneration process. Modified materials must be able to support survival and growth of different cell types. Together, cells and modified biomaterials contribute to the remodeling of the engineered tissue, which affects its performance. This review focuses on the recent advancements in the designs of the scaffolds including the physical and chemical modifications. The last part of this review also discusses designing processes that involve viability of cells.
Keywords: regenerative medicine, scaffold design, physical modification, chemical modification, mechanical properties
Introduction
Loss of tissue function is associated with various rates of morbidity and mortality depending on the degree of severity of the disease. Different therapies exist for diseases affecting different tissues or organs in the body. Therapies can include surgical replacement, surgical resection, and in the case of cancer, chemotherapy and radiotherapy. End-stage diseases or complete organ failure requires surgical transplantation to restore functionality. The high costs of surgeries, the variability in the success of surgeries, the waiting list for organ transplantation, and the limited availability of donors place the patient under socioeconomic pressure. An alternative to the above listed therapies is needed. These limitations have led to the outgrowth of new fields, whereby engineering aspects are incorporated into the principles of surgery and biology.
Regenerative medicine is an emerging field that aims to improve or repair the function of a tissue or an organ. The primary phase of regenerative medicine included the use of cells, preferably the patient’s own cells, in combination with a biocompatible biomaterial as a way of delivery of these cells. Soon after that, the spectrum has broadened to include cell-based therapies, genetics, bioactive molecules, tissue engineering, and clinical approaches. This phase of regenerative medicine included the use of various technologies to precondition the cells–biomaterials composite in order to obtain a mature engineered tissue. These tissues are further characterized using molecular and genetics tools to confirm structure and function. Additionally, regenerative medicine often includes a clinical component. Surgical techniques are applied to evaluate the engineered tissues in vivo before moving toward translational applications. This review will focus on the source of cells used in regenerative medicine, the different designs of biomaterials along with their biomechanical properties, and finally the challenges encountered in regenerative medicine.
Cell Source in Regenerative Medicine
The first step toward success in regenerative medicine is the ability to find a viable cell source. It is of paramount importance to obtain adequate number of functional cells that are able to expand in vitro and that do not cause an immune response upon their implantation. The challenge in obtaining autologous cells resides in the fact that the diseased tissue might not be a good source of cells. This shifts the search to other sources in the body. The use of stem cells in regenerative medicine has gained special attention. Different stem cells are currently being investigated to determine the best candidate. The cells must be immediately available, easy to expand in vitro, and immunocompatible.
Different cells of the body can serve as source for regenerative medicine purposes. Cells obtained from tissue biopsies are usually considered as ideal since they have a determined physiological function. For example, various contractile organs of the body have smooth muscle cells as their functional unit. Therefore, obtaining healthy contractile smooth muscle cells promotes the regeneration process. One of the challenges of using these cells is the ability to maintain their specific phenotype after their expansion in vitro and following their implantation.1,2 One drawback of using tissue-specific cells is the health condition of the tissue itself. Diseased tissues do not provide a viable source of cells and hence do not provide a regenerative capacity.
Neural stem cells are another cell source used in different tissue engineering applications. These cells can be derived from either the central nervous system or the enteric nervous system.3–5 Successful outcomes require complete and adequate innervation of the regenerated tissues. The use of different scaffolds in combination with neural stem cells has demonstrated survival and differentiation of these cells.6,7 Additionally, substrate topography was evaluated as a factor in modulating neural stem cell differentiation.8 Controlling cell differentiation by modifying matrix properties provides a basis for designing scaffolds in regenerative medicine.
Mesenchymal stem cells are considered as the leading stem cell in tissue engineering applications. These cells can be harvested from different sources in the body for in vitro expansion. Mesenchymal stem cells from different sources have also shown comparable characteristics of cell surface markers, and osteogenic and adipogenic differentiation.9
Bone marrow-derived mesenchymal stem cells can be obtained from bone marrow aspirate. The fraction of stem cells found in the aspirate is low. However, these cells can be easily expanded in culture and can differentiate into endodermal and ectodermal cells. Bone marrow mesenchymal stem cells are commonly used in tissue engineering, and protocols for their isolation and differentiation are characterized.10–12
Adipose-derived stem cells are also a practical cell source.13,14 A large quantity of these cells can be obtained through a minimally invasive procedure. Methods of isolation of these cells are available; however, additional optimization is required.15 In previous studies conducted in mice, it has been documented that fat obtained from different depots share the same characteristics; however, the growth rate of the cultured cells decreases with age.16 Adipose-derived stem cells have also been induced to differentiate into Schwann cells in nerve regeneration applications. Differentiated Schwann cells promoted neurite outgrowth when cultured with neurons.17 These cell sources serve as autologous sources, given that the cells can be isolated from the patient, avoiding any immune rejection after their transplantation.
Another source of stem cells is the umbilical cord mesenchymal stem cells.18 Human umbilical cord mesenchymal stem cells isolated from the Wharton’s Jelly in umbilical cords were tested in muscle tissue engineering.19 The cells were embedded in fibrin gel at different mass fractions to determine the optimal composition for cell viability. Myogenesis was demonstrated qualitatively using immunohistochemistry and quantitatively using PCR. Umbilical cord mesenchymal stem cells have also been tested in bone tissue engineering.20 Cells that were seeded onto collagen–calcium phosphate cement scaffolds synthesized minerals, indicating osteogenic differentiation. The advantage of using umbilical cord mesenchymal stem cells is that they are easily accessible at low cost and without invasive procedure. These cells have high proliferative rate. However, the chance of tumorigenesis after their transplantation remains a challenge that requires further investigations. Umbilical cord mesenchymal stem cells have also been demonstrated to be a potential cell source in ocular applications.21 In this study, injection of umbilical cord stem cells into the subretinal space in rats showed response sensitivity of the retina. The authors believe that the umbilical cord stem cells did not differentiate into neurons; rather, they secreted neurotrophic factors that supported rescuing photoreceptors.
Placenta, as other gestational tissues, is usually considered as medical waste following birth. However, a population of mesenchymal stem cells resides in the placenta, which serves as a new potential cell source in regenerative medicine.22–25 The differentiation of placenta-derived mesenchymal stem cells and their application in tissue engineering have been demonstrated.26,27 Placenta can be easily obtained without an invasive procedure and with no risk to the donor. Mesenchymal stem cells can be harvested and expanded in abundance; however, the issue of being immunologically rejected by the body when used in a non-autologous setting is still controversial. Recently, a pilot study aimed to determine the efficacy and safety of using allogeneic stem cells.28 Placenta-derived mesenchymal stem cells were transplanted in patients with type 2 diabetes. Although the study was promising, a larger, more controlled study is required to confirm the results.
Although autologous mesenchymal stem cells have great regenerative potential, their use is limited in cases where the patients have genetic disorders. Advancements in reprogramming technologies have expanded the choice of cell sources. Different cells of the body can be modified into induced pluripotent stem (iPS) cells by changing the expression of certain genes.29 Regardless of the health conditions of the patients, iPS cells can differentiate into different cell types of the body. These cells are considered as patient specific and they provide a model to study disease mechanisms, pathologies, therapeutics, and cure development. Recently, iPS cells were derived from mouse fibroblasts for their use in cartilage tissue engineering.30 Differentiated cells secreted cartilage-specific matrix components, indicating the potential of these cells in repairing cartilage defects. In another study, human iPS cells were used to derive endothelial cells and mesenchymal precursor cells.31 The cells were used in combination to generate a functional blood vessel in vivo. The clinical translation aspect of using iPS cells is the ability to use the patient’s own cells, reprogram them, and reuse them to generate functional tissues.
Embryonic stem cells are another promising cell source in regenerative medicine because of their self-renewal property. These cells have the potential to rapidly expand in vitro and differentiate into almost all types of cells.32,33 Obtaining embryonic stem cells requires sacrificing the embryos that limits their clinical application. To overcome the ethical concerns, studies are being conducted to isolate embryonic stem cells from single blastomeres.34 Immunohistochemical studies demonstrated the differentiation of these cells into cells of all the three germ layers (ectoderm, mesoderm, and endoderm). A clinical trial study was conducted on humans using embryonic stem cells. Cells injected in the subretinal space improved the patients’ vision. There were no signs of abnormal proliferation.
Scaffold Design and Synthesis: Physical and Chemical Modifications
The second component in tissue engineering and regenerative medicine includes the design of a scaffold that acts to supplement the regeneration process or to replace the diseased tissue. The scaffold is a temporary matrix that supports cell attachment, proliferation, and differentiation. An ideal scaffold must have an excellent biocompatibility to ensure cell survival and minimal immune response after implantation. Biodegradability of the scaffold is another important factor in the design of scaffolds. Biodegradability falls in line with adequate mechanical properties of the scaffold. Following implantation, the scaffold must degrade in a timely manner to ensure proper remodeling of the tissue. Highly porous scaffolds are critical for cell infiltration especially when it comes to thick scaffolds where diffusion becomes a limitation. Porosity also plays a role in providing surface area for cell attachment. An ideal scaffold is the outcome of a balance between all these factors. Finally, physical and chemical modifications can be applied to the scaffolds to enhance their bioactivity.
Given all the above listed factors, a perfect design of scaffolds for tissue engineering and regenerative medicine is still a challenge. For example, even though the trachea and the gastrointestinal tract consist of tubular organs, their architecture is very complex and requires sophisticated designing.35,36 Different types of cells, each with a specific alignment, necessitate the synthesis of various scaffolds with precise structures that match the native tissue. Different biomaterials are available to synthesize the scaffolds and different techniques are available to modulate the design of these scaffolds.
Physical modification
The microarchitecture of the tissue is an important element that determines tissue function. Long-term success of the engineered tissues requires the recapitulation of the environment in which the cells must reside. Different approaches to develop scaffolds with specific architectures have been demonstrated. An important factor in the synthesis is the reproducibility in the manufacturing process. Recent reports emphasized the use of computer-aided design (CAD) in engineering scaffolds.37–39 This technique allows fine-tuning the geometry of the scaffold with precise structures.
Our group has previously bioengineered circumferentially aligned intrinsically innervated smooth muscle rings to mimic the circular smooth muscle layer of the gastrointestinal tract.40,41 Tissue culture plates were coated with Sylgard and left to cure. A cylindrical post was fixed in the center of the plate to create the lumen of the engineered tissue. Enteric neural progenitor cells were suspended in collagen/laminin gel and seeded onto the plate. An additional gel layer of smooth muscle cells was added on top of the first one. Within a period of 7 days, the smooth muscle cells aligned concentrically around the central post. Histological evaluation of the engineered tissues revealed smooth muscle alignment and differentiation of the neural progenitor cells into different types of neurons (excitatory and inhibitory neurons). Electromechanical integrity of the smooth muscle cell was demonstrated using potassium chloride in the absence and presence of calcium channel blocker, nifedipine. The presence of muscarinic receptors was also demonstrated using acetylcholine in the absence and presence of muscarinic receptor antagonist, atropine. Neuronal functionality was also demonstrated by electrical field stimulation in the absence and presence of blockers. Although the architecture and functionality of these tissues are established, their mechanical properties are yet to be determined.
In vivo assessment of the bioengineered sphincteric smooth muscle tissues was determined in mice and rat models.42,43 The implanted engineered tissues integrated with the native internal anal sphincter. The tissues were evaluated for their physiological functionality using an organ bath connected to a force transducer set up (Fig. 1). They maintained their myogenic and neurogenic functionality and they became neovascularized. The animals survived throughout the study period and there were no signs of obstruction.
Figure 1.
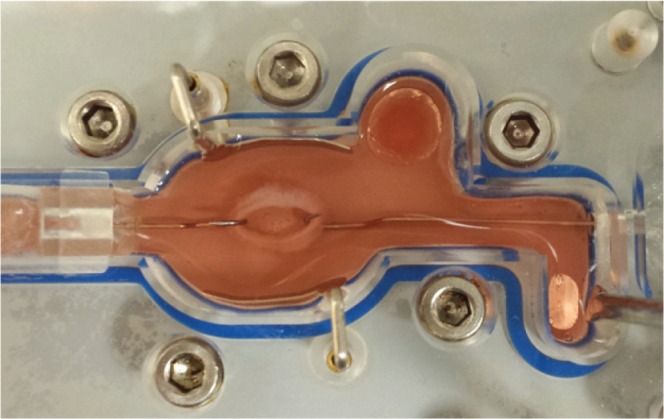
A bioengineered smooth muscle tissue in an organ bath for testing physiological functionality.
In other studies, smooth muscle tissue rings were also engineered on agarose-coated wells.44,45 The cells aggregated around a central post and formed a ring structure. Mechanical properties of the rings were determined at days 8 and 14 post-formation.46 The ultimate tensile strength and ring stiffness were found to be higher in larger rings but decreased as a function of time. However, functionality of the smooth muscle was not evaluated. A correlation between the different mechanical properties of the different ring sizes and the force generated from the smooth muscle is an important factor that needs to be determined in tissue regeneration.
When designing scaffolds in tissue engineering, it is important to keep in mind the physiological condition under which the scaffold will be implanted. Tensile properties, suture retention strength, burst pressure strength, and compliance are among the common properties evaluated for scaffolds. The scaffold must have enough strength not to break following suturing or when exposed to pressure. Compliance ensures that the scaffold does not compress or twist arbitrarily. To guarantee long-term success, these tests must be performed in vitro before implantation. Different designs of scaffolds have been pursued to mimic the architecture of the native tissue. Lee and co-workers engineered a bilayered scaffold for blood vessel replacement using a co-electrospinning technique.47 A polymer blend of polycaprolactone (PCL) and type 1 collagen was electrospun in a two-step process. Small diameter fibers were electrospun as a first layer and then larger diameter fibers were electrospun on top of that layer. This technique allows the seeding of endothelial cells on the small fibers from the luminal side and smooth muscle cells on the large fibers from the external side. The biomechanical and biological properties of the electrospun PCL/collagen scaffolds were also characterized.48 Mechanical properties of the composite scaffolds are within the physiologic range.
Other approaches to control the design of the engineered tissues have attempted to prepare micropatterned thermoresponsive surfaces.49 This technique allowed the formation of aligned cell sheet layers that were placed on top of each other to form two layers. Cells were grown on plates at a specific temperature and then were lifted as a layer at a different temperature. The advantage of using thermoresponsive surfaces is that the cell sheet maintains the extracellular matrix (ECM) components secreted by the cells which are beneficial for cell–cell interaction. Our group has developed a technique to align smooth muscle using a substrate microtopography technique.50 The cells were aligned longitudinally in a similar fashion to the longitudinal smooth muscle layer of the gastrointestinal tract. The cells were lifted as a sheet using laminin/collagen gel. These smooth muscle sheets have also been tested for their physiological functionality. In a different study, PCL substrates with nanowire structures were engineered as matrices for retinal progenitor cells.51 The substrates supported cell proliferation and differentiation as indicated by the expression of photoreceptor markers. Nanowired PCL promoted the migration of the cells into the retinal laminae and resulted in integration with the host retina.
Our group has used tubular chitosan scaffolds as matrices for bioengineered smooth muscle tissues. Chitosan is a natural polymer known for its wide application in tissue engineering. We demonstrated the biocompatibility of chitosan with gut smooth muscle.52 Chitosan scaffolds also promoted the neoinnervation of bioengineered smooth muscle tissues when placed around the scaffold using enteric neural progenitor cells.53 Our scaffolds were prepared using the freeze-drying method as described previously (Fig. 2A). Scanning electron microscopy (SEM) revealed highly porous scaffold with average pore size of around 170 μm (Fig. 2B). Our scaffolds did not leak or burst when pressure was applied; however, they do not possess enough strength to maintain luminal patency. Different techniques are available to improve on the mechanical properties of the chitosan scaffolds. Recently, chitosan fibers were prepared by an extrusion/gelation technique to reinforce the mechanical properties of chitosan-based heart valve scaffolds.54 Tensile properties were improved in the reinforced scaffolds. Additionally, the fibers mechanical properties were modulated following different methods of fabrication.55 The benefit of this technique, where chitosan fibers were used to enhance chitosan scaffold mechanical properties, is that the same material with different structures is used. This reduces the issues of biocompatibility and inconsistency in using other materials.
Figure 2.
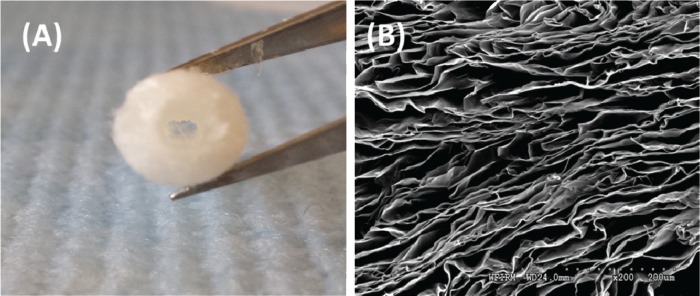
(A) An image of tubular chitosan scaffold prepared using the freeze-drying method. (B) Scanning electron microscopy (SEM) characterization of the scaffold reveals a highly porous scaffold.
Chemical modifications
In addition to changing the physical characteristics of the scaffolds, chemical modifications are available approaches to enhance the properties of the material. In designing a blood vessel graft, a gradient nanofibrous tubular scaffold was synthesized by electrospinning the polymer solution at different flow rates.56 Heparin was conjugated with the nanofibers as an antithrombogenic factor. The gradient scaffolds were then loaded with VEGF in a way that heparin–VEGF level was higher on the luminal side and lower on the external side. Gradient heparin–VEGF enhanced the release of the loaded VEGF from the scaffold and promoted cell attachment. Controlling the release of VEGF helps in tuning the attachment and function of the cells seeded on the scaffold.
In a recent study, a microporous fibrin scaffold was designed with open microchannels and adequate mechanical stiffness in an attempt to engineer cardiac tissue.57 The design of the scaffold allows the seeding of cardiomyocytes, endothelial cells, and fibroblasts in a similar alignment as the native tissue. The architecture of the scaffold also facilitated better cell seeding and survival. The tri-cell seeded scaffolds promoted the formation of a prevascular network with aligned cardiac tissue.
The disadvantage with the use of synthetic polymers is their inability to support cell attachment. Chemical surface modification can improve the biocompatibility of materials and enhance cell growth. Electrospun poly(L-lactide) scaffolds were treated with Ar/NH3 plasma to introduce amine groups to the scaffold.58 Surface characterization of the scaffolds using atomic force microscopy (AFM) and X-ray photoelectron spectroscopy (XPS) revealed an increase in hydrophilicity of the material without affecting the structure of the fibers. Surface modification promoted cell attachment, spreading, and infiltration. In another study, addition of gelatin type B and fibronectin to poly(ε-caprolactone) improved the biological behavior of the scaffold and enhanced cell colonization and ECM deposition.59 Different strategies to modify the surface of scaffolds exist. These strategies can either be applied individually or in combination. It is critical to determine the necessary modification to enhance the activity of the scaffold.
Chemical modification of the scaffold proved to be as critical as physical modification. A polymer solution composed of poly-L-lactic acid (PLLA), poly-(α,β)-DL-aspartic acid (PAA), type I and type III collagen was used to electrospin nanofibrous scaffolds.60 Addition of functional groups to the scaffold made it more hydrophilic as demonstrated by water contact angle measurement. This characteristic allowed more cell attachment, increased the rate of proliferation, and increased secretion of ECM proteins. Polyethylene glycol hydrogels were evaluated as injectable biomaterials.61 The hydrogels were functionalized with laminin at different ratios to assess the attachment of nucleus pulposus cells. The stiffness of the hydrogels can be modulated by changing the concentration of the polyethylene glycol–laminin conjugate. This study supports the idea that manipulating different features in the design of the material provides an optimal scaffold/carrier that can be used in tissue regeneration.
In other tissue regeneration applications, chemical surface modification is used to manipulate cell differentiation. Adhesion peptides such as fibronectin and laminin and neurotrophic factors were immobilized on polymer substrates to evaluate the differentiation of neural stem cells.62 The modified surface was characterized by AFM and SEM to look at morphology and roughness. Wettability of the immobilized surface was evaluated by water contact angle measurement and revealed increase in hydrophilicity. Additionally, immobilized surfaces enhanced neuronal differentiation and proliferation. On the other hand, changing the composition of the ECM components has an influence on differentiation of neural stem cells into specific types of neurons. Our group has demonstrated the ability to differentiate enteric neural progenitor cells into specific types of neurons using different combination of ECM components.63 The composition of the substrates modulated the extent of neuronal and glial differentiation. A higher neuronal population was observed when enteric neural progenitor cells were seeded onto composite mixtures (Collagen IV, laminin and heparan sulfate) than glial population.
Growth factor delivery is also a technique used for tissue regeneration. The activity of the growth factor must be timely and spatially controlled. Delivery can be done by incorporating growth factors onto scaffolds or through carriers. In order for the growth factor to deliver the correct message, it must diffuse through the ECM and bind to the target cell.64 To ensure adequate delivery and controlled release of growth factors, modified polymeric matrices are used to increase the therapeutic efficiency of these factors. Growth factor delivery has dual roles; it controls both the efficacy of the factor and the response of the cell through chemotaxis. Cell migration and differentiation are complex mechanisms that depend on the spatial distribution of the growth factor. Scaffolds can be designed to take this component into account. The impact of chemotactic gradients of different growth factors on stem cell migration is critical in tissue engineering applications.65 Spatial patterns of growth factors provided migratory cues for the cells away from their source. This becomes critical when cells have to migrate over a long distance. Growth factors including platelet-derived growth factors (PDGF) are known to regulate chemotaxis. In bone regeneration, scaffolds loaded with PDGF favor bone cell migration and help in accelerating the regeneration process.66 The challenge remains in determining the optimal dose of growth factor. It is also critical to take into account the release kinetics of the growth factor. Recently, scaffolds are being designed to allow sequential delivery of multiple factors.67,68 Different bone morphogenic proteins were loaded into different carriers and then incorporated into scaffolds. The sequential delivery approach enhanced cell differentiation and provided a better control over the release kinetics. Recently, Simson et al mixed chondroitin sulfate-bone marrow adhesive hydrogel with bone morphogenetic-2 protein as an attempt to regenerate cartilage tissue.69 The carrier allowed the chondrocytes to maintain their phenotype and to produce sulfated glycosaminoglycans. The efficacy of this system is demonstrated by the retention and activity of the growth factor during the experiment and by the adhesive properties of chondroitin sulfate. A challenge that the authors have brought up to their system is the stability of the carriers, which is a pre-requisite for translational purposes.
Cell Viability in the Scaffold: Design Process
The long-term success of implanted scaffolds is dependent on the delivery of an adequate number of viable and functional cells required to repair damaged tissues. As the scaffolds or the grafts get bigger, the hypoxic regions within the scaffolds limit the performance of the cells to support regeneration. Nutrients, and most critically oxygen, need to be made available for cells post-transplantation. Recently, Wang et al developed oxygen-enriched scaffolds to enhance cell survival and function following implantation.70
A major challenge in tissue engineering application is the limited diffusion ability of oxygen in the scaffold. Scaffolds design must ensure that cells have access to nutrients until neo-vascularization occurs. Uneven distribution of cells is associated with different oxygen distribution in the scaffolds. Improved expression of markers, enhanced cell functions, and maintenance of cell phenotypes are the result of uniform distribution of viable cells in the scaffolds.
Mathematical models were developed to predict the oxygen gradients within engineered scaffolds, taking into account scaffold dimensions and cell function.71–74 These models can be used to organize design criteria. Cells within scaffolds access oxygen mainly through diffusion. Determining the oxygen gradients will allow optimizing the cell seeding density to maximize viability. Studies have shown that high cellular viability correlates with higher oxygen concentration within the scaffolds. Cells at the surface of the scaffolds exhibited better viability than cells deeper in the scaffold, and this is attributed to the limited diffusivity of oxygen.75
Diffusion reaction models based on diffusive oxygen transport are commonly applied to correlate the distribution of oxygen within a construct cultured to the distribution of cell density and viability. These models assume that there is no convective flux within the scaffold, the oxygen diffusion coefficient is constant and that there is homogenous distribution of cells in the scaffold. The change in oxygen concentration is described by the balance of oxygen diffusion through the scaffold and the rate of oxygen consumption by the cells. Oxygen is considered to be consumed by the cells according to the Michaelis–Menten kinetics. Oxygen consumption rate by the cells is determined and cell viability will be evaluated.76 Oxygen uptake is dependent on the cell type. Defining boundary conditions, oxygen profile within the scaffold can be modeled and correlated with cell viability. Scaffolds placed in static conditions behaved differently from scaffolds exposed to flow. The profile of oxygen concentration in static conditions showed a linear decrease as a function of depth. However, flow conditions improved the oxygen concentration in the scaffold.75
These models can become more complicated when different factors are accounted for in the design of the scaffolds. In addition to oxygen, availability of other nutrients can also be modeled. Deviations may occur during long-term culture because of the remodeling of the scaffold by ECM deposition by the cells.77 Therefore, changes in nutrient diffusivities are additional factors that must be taken into account in these models.
Closing Remarks
Despite the advances in technologies and techniques used in tissue engineering and regenerative medicine, there are more questions that need to be answered. Design of scaffolds is an essential feature in regenerative medicine. It dictates cell behavior and function. Physical and chemical modifications exist to enhance the bioactivity of the scaffolds. Although different designs have proven to be beneficial, further optimization is needed. It is critical that cells maintain their phenotypic characteristics when seeded on the scaffold. Maximum cell viability is also crucial for long-term success of the implant. Additionally, design of the scaffold affects tissue remodeling and performance after implantation. Vascularization is another challenging task that must be taken into consideration in tissue regeneration. Several studies are currently being conducted to precondition tissues prior to their implantation to maximize vascularization. The field of regenerative medicine is still in its early stages of emergence where there is still room for optimization.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: KNB and EZ. Contributed to the writing of the manuscript: KNB and EZ. Made critical revisions and approved final version: KNB and EZ. All authors reviewed and approved the final manuscript.
DISCLOSURES AND ETHICS
This paper was subject to independent, expert peer review by a minimum of two blind peer reviewers. All editorial decisions were made by the independent academic editor. All authors have provided signed confirmation of their compliance with ethical and legal obligations including (but not limited to) use of any copyrighted material, compliance with ICMJE authorship and competing interests disclosure guidelines and, where applicable, compliance with legal and ethical guidelines on human and animal research participants. Provenance: the authors were invited to submit this paper.
ACADEMIC EDITOR: Kayvan Najarian, Editor in Chief
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
FUNDING: This work was supported by NIH RO1 DK 071614. Funding sources have not influenced the content of the paper.
REFERENCES
- 1.Halayko AJ, Camoretti-Mercado B, Forsythe SM, et al. Divergent differentiation paths in airway smooth muscle culture: induction of functionally contractile myocytes. Am J Physiol. 1999;276(1 pt 1):L197–L206. doi: 10.1152/ajplung.1999.276.1.L197. [DOI] [PubMed] [Google Scholar]
- 2.Brittingham J, Phiel C, Trzyna WC, Gabbeta V, McHugh KM. Identification of distinct molecular phenotypes in cultured gastrointestinal smooth muscle cells. Gastroenterology. 1998;115(3):605–617. doi: 10.1016/s0016-5085(98)70140-4. [DOI] [PubMed] [Google Scholar]
- 3.Suárez-Rodríguez R, Belkind-Gerson J. Cultured nestin-positive cells from postnatal mouse small bowel differentiate ex vivo into neurons, glia, and smooth muscle. Stem Cells. 2004;22(7):1373–1385. doi: 10.1634/stemcells.2003-0049. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer K-H, Hagl CI, Rauch U. Differentiation of neurospheres from the enteric nervous system. Pediatr Surg Int. 2003;19(5):340–344. doi: 10.1007/s00383-003-1007-4. [DOI] [PubMed] [Google Scholar]
- 5.Scanga VI, Goraltchouk A, Nussaiba N, Shoichet MS, Morshead CM. Biomaterials for neural-tissue engineering-Chitosan supports the survival, migration, and differentiation of adult-derived neural stem and progenitor cells. Can J Chem. 2010;88(3):277–287. [Google Scholar]
- 6.Nisbet DR, Rodda AE, Horne MK, Forsythe JS, Finkelstein DI. Neurite infiltration and cellular response to electrospun polycaprolactone scaffolds implanted into the brain. Biomaterials. 2009;30(27):4573–4580. doi: 10.1016/j.biomaterials.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Leipzig ND, Wylie RG, Kim H, Shoichet MS. Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials. 2011;32(1):57–64. doi: 10.1016/j.biomaterials.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 8.Lim SH, Liu XY, Song H, Yarema KJ, Mao HQ. The effect of nanofiber-guided cell alignment on the preferential differentiation of neural stem cells. Biomaterials. 2010;31(34):9031–9039. doi: 10.1016/j.biomaterials.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33(11):1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Alves da Silva ML, Martins A, Costa-Pinto AR, et al. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J Tissue Eng Regen Med. 2011;5(9):722–732. doi: 10.1002/term.372. [DOI] [PubMed] [Google Scholar]
- 11.Tian H, Bharadwaj S, Liu Y, et al. Myogenic differentiation of human bone marrow mesenchymal stem cells on a 3D nano fibrous scaffold for bladder tissue engineering. Biomaterials. 2010;31(5):870–877. doi: 10.1016/j.biomaterials.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Izal I, Aranda P, Sanz-Ramos P, et al. Culture of human bone marrow-derived mesenchymal stem cells on of poly(L-lactic acid) scaffolds: potential application for the tissue engineering of cartilage. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1737–1750. doi: 10.1007/s00167-012-2148-6. [DOI] [PubMed] [Google Scholar]
- 13.Katz AJ, Tholpady A, Tholpady SS, Shang H, Ogle RC. Cell surface and transcriptional characterization of human adipose-derived adherent stromal (hADAS) cells. Stem Cells. 2005;23(3):412–423. doi: 10.1634/stemcells.2004-0021. [DOI] [PubMed] [Google Scholar]
- 14.Anghileri E, Marconi S, Pignatelli A, et al. Neuronal differentiation potential of human adipose-derived mesenchymal stem cells. Stem Cells Dev. 2008;17(5):909–916. doi: 10.1089/scd.2007.0197. [DOI] [PubMed] [Google Scholar]
- 15.Sterodimas A, de Faria J, Nicaretta B, Pitanguy I. Tissue engineering with adipose-derived stem cells (ADSCs): current and future applications. J Plast Reconstr Aesthet Surg. 2010;63(11):1886–1892. doi: 10.1016/j.bjps.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Sowa Y, Imura T, Numajiri T, Nishino K, Fushiki S. Adipose-derived stem cells produce factors enhancing peripheral nerve regeneration: influence of age and anatomic site of origin. Stem Cells Dev. 2012;21(11):1852–1862. doi: 10.1089/scd.2011.0403. [DOI] [PubMed] [Google Scholar]
- 17.Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207(2):267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Surbek DV, Holzgreve W, Jansen W, et al. Quantitative immunophenotypic characterization, cryopreservation, and enrichment of second- and third-trimester human fetal cord blood hematopoietic stem cells (progenitor cells) Am J Obstet Gynecol. 1998;179(5):1228–1233. doi: 10.1016/s0002-9378(98)70137-1. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Xu HH, Zhou H, Weir MD, Chen Q, Trotman CA. Human umbilical cord stem cell encapsulation in novel macroporous and injectable fibrin for muscle tissue engineering. Acta Biomater. 2013;9(1):4688–4697. doi: 10.1016/j.actbio.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thein-Han W, Xu HH. Collagen–calcium phosphate cement scaffolds seeded with umbilical cord stem cells for bone tissue engineering. Tissue Eng Part A. 2011;17:23–24. 2943–2954. doi: 10.1089/ten.tea.2010.0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25(3):602–611. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- 22.Murphy SV, Atala A. Amniotic fluid and placental membranes: unexpected sources of highly multipotent cells. Semin Reprod Med. 2013;31(1):62–68. doi: 10.1055/s-0032-1331799. [DOI] [PubMed] [Google Scholar]
- 23.Semenov OV, Koestenbauer S, Riegel M, et al. Multipotent mesenchymal stem cells from human placenta: critical parameters for isolation and maintenance of stemness after isolation. Am J Obstet Gynecol. 2010;202(2):e191–e193. doi: 10.1016/j.ajog.2009.10.869. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Zhang W, Jiang X, Mao N. Human-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res. 2007;330(3):437–446. doi: 10.1007/s00441-007-0504-5. [DOI] [PubMed] [Google Scholar]
- 25.Parolini O, Alviano F, Bergwerf I, et al. Toward cell therapy using placenta-derived cells: disease mechanisms, cell biology, preclinical studies, and regulatory aspects at the round table. Stem Cells Dev. 2010;19(2):143–154. doi: 10.1089/scd.2009.0404. [DOI] [PubMed] [Google Scholar]
- 26.Hsu SH, Huang TB, Cheng SJ, et al. Chondrogenesis from human placenta-derived mesenchymal stem cells in three-dimensional scaffolds for cartilage tissue engineering. Tissue Eng Part A. 2011;17:11–12. 1549–1560. doi: 10.1089/ten.TEA.2010.0419. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Tong A, Zhou L, Fang F, Guo G. Osteogenic differentiation of human placenta-derived mesenchymal stem cells (PMSCs) on electrospun nanofiber meshes. Cytotechnology. 2012;64(6):701–710. doi: 10.1007/s10616-012-9450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang R, Han Z, Zhuo G, et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med. 2011;5(1):94–100. doi: 10.1007/s11684-011-0116-z. [DOI] [PubMed] [Google Scholar]
- 29.Bellin M, Marchetto MC, Gage FH, Mummery CL. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol. 2012;13(11):713–726. doi: 10.1038/nrm3448. [DOI] [PubMed] [Google Scholar]
- 30.Diekman BO, Christoforou N, Willard VP, et al. Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proc Nat Acad Sci U S A. 2012;109(47):19172–19177. doi: 10.1073/pnas.1210422109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samuel R, Daheron L, Liao S, et al. Generation of functionally competent and durable engineered blood vessels from human induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2013;110(31):12774–12779. doi: 10.1073/pnas.1310675110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kehat I, Kenyagin-Karsenti D, Snir M, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108(3):407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A. 2003;100(22):12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444(7118):481–485. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 35.Del Gaudio C, Baiguera S, Ajalloueian F, Bianco A, Macchiarini P. Are synthetic scaffolds suitable for the development of clinical tissue-engineered tubular organs? J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34883. [DOI] [PubMed] [Google Scholar]
- 36.Bitar KN, Zakhem E. Tissue engineering and regenerative medicine as applied to the gastrointestinal tract. Curr Opin Biotechnol. 2013;24(5):909–915. doi: 10.1016/j.copbio.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fielding GA, Bandyopadhyay A, Bose S. Effects of silica and zinc oxide doping on mechanical and biological properties of 3D printed tricalcium phosphate tissue engineering scaffolds. Dent Mater. 2012;28(2):113–122. doi: 10.1016/j.dental.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ovsianikov A, Deiwick A, Van Vlierberghe S, et al. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules. 2011;12(4):851–858. doi: 10.1021/bm1015305. [DOI] [PubMed] [Google Scholar]
- 39.Gauvin R, Chen YC, Lee JW, et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33(15):3824–3834. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somara S, Gilmont RR, Dennis RG, Bitar KN. Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology. 2009;137(1):53–61. doi: 10.1053/j.gastro.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 41.Hecker L, Baar K, Dennis RG, Bitar KN. Development of a three- dimensional physiological model of the internal anal sphincter bioengineered in vitro from isolated smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 2005;289(2):G188–G196. doi: 10.1152/ajpgi.00335.2004. [DOI] [PubMed] [Google Scholar]
- 42.Raghavan S, Gilmont RR, Miyasaka EA, et al. Successful implantation of bioengineered, intrinsically innervated, human internal anal sphincter. Gastroenterology. 2011;141(1):310–319. doi: 10.1053/j.gastro.2011.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilmont RR, Raghavan S, Somara S, Bitar KN. Bioengineering of physiologically functional intrinsically innervated human internal anal sphincter constructs. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2013.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adebayo O, Hookway TA, Hu JZ, Billiar KL, Rolle MW. Self-assembled smooth muscle cell tissue rings exhibit greater tensile strength than cell-seeded fibrin or collagen gel rings. J Biomed Mater Res A. 2013;101(2):428–437. doi: 10.1002/jbm.a.34341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gwyther TA, Hu JZ, Billiar KL, Rolle MW. Directed cellular self-assembly to fabricate cell-derived tissue rings for biomechanical analysis and tissue engineering. J Vis Exp. 2011;(57):e3366. doi: 10.3791/3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gwyther TA, Hu JZ, Christakis AG, et al. Engineered vascular tissue fabricated from aggregated smooth muscle cells. Cells Tissues Organs. 2011;194(1):13–24. doi: 10.1159/000322554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ju YM, Choi JS, Atala A, Yoo JJ, Lee SJ. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials. 2010;31(15):4313–4321. doi: 10.1016/j.biomaterials.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Lee SJ, Liu J, Oh SH, Soker S, Atala A, Yoo JJ. Development of a composite vascular scaffolding system that withstands physiological vascular conditions. Biomaterials. 2008;29(19):2891–2898. doi: 10.1016/j.biomaterials.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi H, Shimizu T, Nakayama M, Yamato M, Okano T. The use of anisotropic cell sheets to control orientation during the self-organization of 3D muscle tissue. Biomaterials. 2013;34(30):7372–7380. doi: 10.1016/j.biomaterials.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 50.Raghavan S, Lam MT, Foster LL, et al. Bioengineered three-dimensional physiological model of colonic longitudinal smooth muscle in vitro. Tissue Eng Part C Methods. 2010;16(5):999–1009. doi: 10.1089/ten.tec.2009.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Redenti S, Tao S, Yang J, et al. Retinal tissue engineering using mouse retinal progenitor cells and a novel biodegradable, thin-film poly (e-caprolactone) nanowire scaffold. J Ocul Biol Dis Infor. 2008;1(1):19–29. doi: 10.1007/s12177-008-9005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zakhem E, Raghavan S, Gilmont RR, Bitar KN. Chitosan-based scaffolds for the support of smooth muscle constructs in intestinal tissue engineering. Biomaterials. 2012;33(19):4810–4817. doi: 10.1016/j.biomaterials.2012.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zakhem E, Raghavan S, Bitar KN. Neo-innervation of a bioengineered intestinal smooth muscle construct around chitosan scaffold. Biomaterials. 2014;35(6):1882–1889. doi: 10.1016/j.biomaterials.2013.11.049. [DOI] [PubMed] [Google Scholar]
- 54.Albanna MZ, Bou-Akl TH, Walters HL, III, Matthew HW. Improving the mechanical properties of chitosan-based heart valve scaffolds using chitosan fibers. J Mech Behav Biomed Mater. 2012;5(1):171–180. doi: 10.1016/j.jmbbm.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Albanna MZ, Bou-Akl TH, Blowytsky O, Walters HL, III, Matthew HW. Chitosan fibers with improved biological and mechanical properties for tissue engineering applications. J Mech Behav Biomed Mater. 2013;20:217–226. doi: 10.1016/j.jmbbm.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Du F, Wang H, Zhao W, et al. Gradient nanofibrous chitosan/poly varepsiloncaprolactone scaffolds as extracellular microenvironments for vascular tissue engineering. Biomaterials. 2012;33(3):762–770. doi: 10.1016/j.biomaterials.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 57.Thomson KS, Korte FS, Giachelli CM, Ratner BD, Regnier M, Scatena M. Prevascularized microtemplated fibrin scaffolds for cardiac tissue engineering applications. Tissue Eng Part A. 2013;19:7–8. 967–977. doi: 10.1089/ten.tea.2012.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng Q, Lee BL, Komvopoulos K, Yan Z, Li S. Plasma surface chemical treatment of electrospun poly(L-lactide) microfibrous scaffolds for enhanced cell adhesion, growth, and infiltration. Tissue Eng Part A. 2013;19:9–10. 1188–1198. doi: 10.1089/ten.tea.2011.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Declercq HA, Desmet T, Berneel EE, Dubruel P, Cornelissen MJ. Synergistic effect of surface modification and scaffold design of bioplotted 3-D polyepsilon-caprolactone scaffolds in osteogenic tissue engineering. Acta Biomater. 2013;9(8):7699–7708. doi: 10.1016/j.actbio.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Ravichandran R, Venugopal JR, Sundarrajan S, Mukherjee S, Sridhar R, Ramakrishna S. Composite poly-L-lactic acid/poly-(alpha,beta)-DL-aspartic acid/collagen nanofibrous scaffolds for dermal tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2012;32(6):1443–1451. doi: 10.1016/j.msec.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 61.Francisco AT, Mancino RJ, Bowles RD, et al. Injectable laminin-functionalized hydrogel for nucleus pulposus regeneration. Biomaterials. 2013;34(30):7381–7388. doi: 10.1016/j.biomaterials.2013.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang K, Lee JS, Kim J, et al. Polydopamine-mediated surface modification of scaffold materials for human neural stem cell engineering. Biomaterials. 2012;33(29):6952–6964. doi: 10.1016/j.biomaterials.2012.06.067. [DOI] [PubMed] [Google Scholar]
- 63.Raghavan S, Gilmont RR, Bitar KN. Neuroglial differentiation of adult enteric neuronal progenitor cells as a function of extracellular matrix composition. Biomaterials. 2013;34(28):6649–6658. doi: 10.1016/j.biomaterials.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miller ED, Li K, Kanade T, Weiss LE, Walker LM, Campbell PG. Spatially directed guidance of stem cell population migration by immobilized patterns of growth factors. Biomaterials. 2011;32(11):2775–2785. doi: 10.1016/j.biomaterials.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park YJ, Lee YM, Park SN, Sheen SY, Chung CP, Lee SJ. Platelet derived growth factor releasing chitosan sponge for periodontal bone regeneration. Biomaterials. 2000;21(2):153–159. doi: 10.1016/s0142-9612(99)00143-x. [DOI] [PubMed] [Google Scholar]
- 67.Basmanav FB, Kose GT, Hasirci V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials. 2008;29(31):4195–4204. doi: 10.1016/j.biomaterials.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 68.Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N, Hasirci V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials. 2009;30(21):3551–3559. doi: 10.1016/j.biomaterials.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 69.Simson JA, Strehin IA, Lu Q, Uy MO, Elisseeff JH. An adhesive bone marrow scaffold and bone morphogenetic-2 protein carrier for cartilage tissue engineering. Biomacromolecules. 2013;14(3):637–643. doi: 10.1021/bm301585e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Qi F, Zhu S, et al. A synthetic oxygen carrier in fibrin matrices promotes sciatic nerve regeneration in rats. Acta Biomater. 2013;9(7):7248–7263. doi: 10.1016/j.actbio.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 71.Zhou S, Cui Z, Urban JP. Nutrient gradients in engineered cartilage: metabolic kinetics measurement and mass transfer modeling. Biotechnol Bioeng. 2008;101(2):408–421. doi: 10.1002/bit.21887. [DOI] [PubMed] [Google Scholar]
- 72.Rutkowski GE, Heath CA. Development of a bioartificial nerve graft. I. Design based on a reaction-diffusion model. Biotechnol Prog. 2002;18(2):362–372. doi: 10.1021/bp020300f. [DOI] [PubMed] [Google Scholar]
- 73.Malda J, Rouwkema J, Martens DE, et al. Oxygen gradients in tissue-engineered PEGT/PBT cartilaginous constructs: measurement and modeling. Biotechnol Bioeng. 2004;86(1):9–18. doi: 10.1002/bit.20038. [DOI] [PubMed] [Google Scholar]
- 74.Stabler C, Fraker C, Pedraza E, Constantinidis I, Sambanis A. Modeling and in vitro and in vivo characterization of a tissue engineered pancreatic substitute. Journal of Combinatorial Optimization. 2009;17(1):54–73. [Google Scholar]
- 75.Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93(2):332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Wilshaw SP, Korossis S, Fisher J, Jin Z, Ingham E. Factors influencing the oxygen consumption rate of aortic valve interstitial cells: application to tissue engineering. Tissue Eng Part C Methods. 2009;15(3):355–363. doi: 10.1089/ten.tec.2008.0415. [DOI] [PubMed] [Google Scholar]
- 77.Obradovic B, Meldon JH, Freed LE, Vunjak-Novakovic G. Glycosaminoglycan deposition in engineered cartilage: experiments and mathematical model. AIChE J. 2000;46(9):1860–1871. [Google Scholar]


