Figure 1.
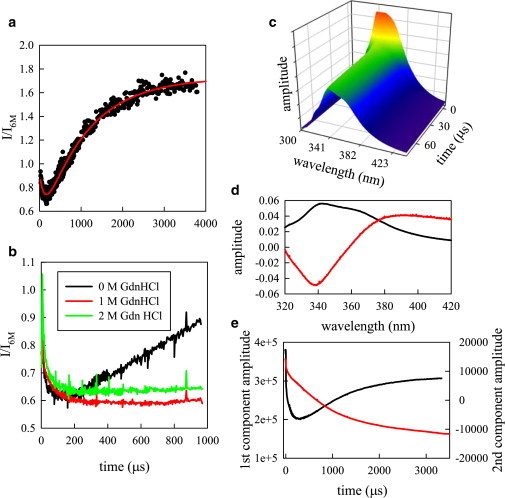
Folding kinetics of protein G as measured by Trp fluorescence. (A) Fluorescence in 0 M GdnHCl scaled to the unfolded protein (in 6 M GdnHCl) as measured by the photon counter (black points). Measurements made with three flow rates (1, 0.5, and 0.125 m/s) are overlaid. The red line is a fit to two exponentials with opposing amplitudes. (B) Fluorescence scaled to the unfolded protein for three final concentrations of denaturant. When fitted to a single exponential, the fast decay time constants are 74 μs (red) and 42 μs (green). (C) Time-resolved fluorescence emission as measured by the spectrograph and charged coupled device (CCD). (D) Two most significant spectral components determined by singular value decomposition (SVD). The first component (black line) is the average spectrum of all measurements and the second component (red line) is the blue shift of the spectrum as the protein folds. (E) Time resolved amplitudes of the first (black) and second (red) SVD components. To see this figure in color, go online.
