Abstract
Context and Objective:
Statin treatment, apart from its hypolipidemic action has proven its antimicrobial activity by improving the survival rate of patients with severe systemic bacterial infections. Periodontitis is an inflammatory disorder of tooth supporting structures caused by a group of specific microorganisms. The objective of the present study was to determine the antimicrobial activity of pure simvastatin drug against the primary periodontal pathogens.
Materials and Methods:
Minimum inhibitory concentration (MIC) was determined against Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans using serial dilution method.
Results:
MIC of simvastatin against P. gingivalis was 2 μg/ml and A. actinomycetemcomitans was found to be <1 μg/ml which requires further dilutions to determine the exact value.
Conclusions:
Data suggests a potent antimicrobial activity of simvastatin against both A. actinomycetemcomitans and P gingivalis. Hence simvastatin can be prescribed as a dual action drug in patients with both hyperlipidemia and periodontal disease.
Keywords: Actinobacillus actinomycetemcomitans, minimum inhibitory concentration, Porphyromonas gingivalis, simvastatin
Introduction
Periodontal diseases are among the most widespread oral bacterial diseases of mankind that affects 10-15% of the world's population eventually leads to tooth loss, if left untreated. Although bacteria belonging to more than 630 different taxa exist in the oral cavity, only 10-15 bacterial species are recognized as potential periodontal pathogens. Of them, Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis are recognized as the major pathogens for initiation and progression of destruction of tooth supporting structures. A. actinomycetemcomitans is a nonmotile, capnophilic, Gram-negative, coccobacillus, and a facultative anaerobe. It produces a number of potentially damaging metabolites including leukotoxin, cytolethal distending toxin. P. gingivalis is a nonmotile, asaccharolytic, Gram-negative coccobacillus, and an obligate anaerobe that produces large array of virulence factors such as leukotoxin and lipopolysaccharides. Longitudinal and retrospective studies have demonstrated an increased risk of periodontal breakdown in A. actinomycetemcomitans and P. gingivalis positive sites and better posttreatment results in their absence.[1]
The logical initiation to control these organisms by mechanical means resulted in minimal long-term effects due to their ability to invade gingival epithelial cells in vitro and buccal epithelial cells in vivo.[2,3] Hence, adjunctive antimicrobial therapy along with appropriate mechanical therapy helped in a significant reduction of both A. actinomycetemcomitans and P. gingivalis.[4] Table 1 lists the characteristics of few important antimicrobials that are commonly used to treat periodontitis.[5] Each antimicrobial agent has its own characteristic, which plays an important role in its action against specific bacteria.
Table 1.
Characteristics of commonly used antimicrobials to treat periodontitis
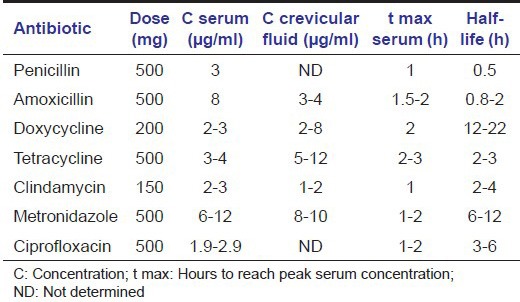
Minimum inhibitory concentration (MIC) is the lowest concentration of a drug that inhibits the visible growth of test organism. In vitro detection of MIC of a drug against pathogens acts as a guideline for its in vivo application. Clinicians use MIC scores to choose appropriate antibiotics to patients with specific infections and to identify an effective dose of the drug.[6]
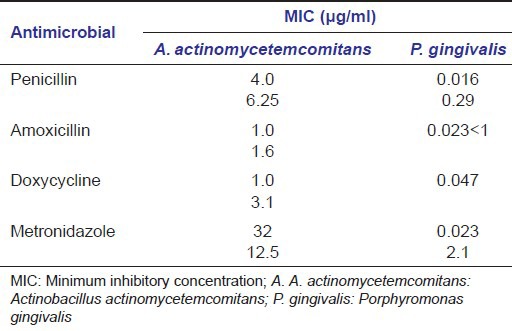
Considering the pathogenic potential of A. actinomycetemcomitans and P. gingivalis, the MIC of various commonly used antimicrobials has been investigated. Table 2 represents few reports with variable results, which can be attributed to change in temperature, inoculum size, pH, growth medium, and specific strain of microorganism during in vitro evaluation.[7]
Table 2.
MIC values of A. actinomycetemcomitans and P. gingivalis to selected antimicrobial agent
Now-a-days cardiovascular diseases (CVDs) rank among the leading causes of death. Nearly, a two-fold increased risk of coronary heart disease was observed in individuals with periodontal disease.[8] On examination of 50 carotid endarterectomized human specimens, 26% were positive for P. gingivalis and 18% were positive for A. actinomycetemcomitans.[9] These findings supports the therapeutic end points to eliminate or lower the number of these specific pathogenic microbes to prevent the initiation, progression and recurrence of periodontitis and in turn CVD.
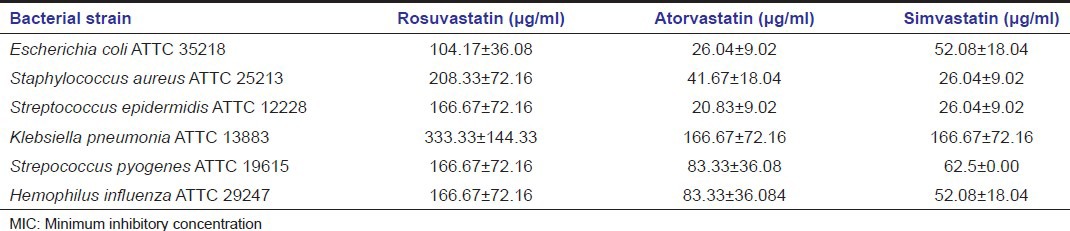
These days’ statins are one of the most commonly prescribed drugs to control the serum cholesterol levels to reduce the risk of CVD. Apart from this, statins have been ascribed for showing various additional pleiotropic effects such as anti-inflammatory, immune modulatory, antioxidant, and anti-carcinogenic properties.[10] Systemic antimicrobial effects of statins have also been proved.[11] MIC values of different statins were evaluated against various nonperiodontal pathogens.[12,13] The results showed a better antibacterial activity of simvastatin on the majority of tested bacteria [Table 3].
Table 3.
MICs (μg/ml) of different statins against few systemic bacteria
Simvastatin, a type I statin is obtained by natural fermentation of fungus, Aspergillus terrus.[14] It is marketed in the form of tablets (10-80 mg) under the trade name Zocor®. Present scientific literature on simvastatin revealed its good applicability in the field of periodontics as a local drug delivery agent due to its anti-inflammatory and bone regenerative properties.[15]
Although simvastatin's antimicrobial activity was proven on many systemic pathogenic bacteria,[11] until date no studies have been carried out on its activity against specific periodontal pathogens. Hence, the present in vitro study was aimed to find out the MIC of pure simvastatin drug that can be safely and effectively administered as an antimicrobial agent on specific periodontal pathogens, P. gingivalis and A. actinomycetemcomitans.
Materials and Methods
Pure simvastatin drug (powder form) was obtained from Dr. Reddy's laboratories, Hyderabad, India. It was certified to be free from any form of bacteria, yeast or mold by the manufacturer after microbial analysis.
Preparation of bacterial suspension
From the maintained frozen stock cultures of A. actinomycetemcomitans (ATCC No-25586) and P. gingivalis (ATCC No-33277) (American type culture collection, Manassas, VA, USA), small quantity of cells were recovered and subcultured. Brain heart infusion (BHI) broth was used as the culture medium to support the growth of bacteria. This culture was transferred into the tubes containing 2 ml of the BHI medium to get culture suspension of A. actinomycetemcomitans and P. gingivalis respectively. The selected test bacterial strains were adjusted for 0.5 McFarland turbidity standards (108 colony forming units/ml) to check the MIC of simvastatin against them.[16]
Determination of minimum inhibitory concentrations
In the present study, serial tube dilution technique was followed based on the guidelines of the Clinical and Laboratory Standards Institute due to its ability to determine antimicrobial activity of the drug along with its MIC values.[17] Stock solution was prepared by dissolving the pure drug in dimethyl sulfoxide at a concentration of 10 mg/ml. In the initial tube 100 μl of stock solution was added into the 300 μl of BHI broth to make a volume of 400 μl, from which nine serial dilutions were prepared in separate test tubes containing 200 μl of BHI broth. To each serially diluted tube, 200 μl of the previously prepared bacterial suspension was added and incubated for 24 h in an anaerobic jar at 37°C and observed for turbidity which indicates the growth of the organisms. The turbidity in each tube was compared with a positive control, which contained only the pure bacterial culture. The least concentration of the drug in the tube, which does not show any turbidity, was considered as MIC of the drug for that particular test organism.
Results
In vitro antibacterial activity
In the present study, both A. actinomycetemcomitans and P. gingivalis were sensitive to pure simvastatin drug. P. gingivalis was sensitive until 2 μg/ml dilution and showed resistance to further dilution by illuminating its MIC. But for A. actinomycetemcomitans, the performed dilutions could not show any visible growth of the organism; hence, MIC value was considered to be <1 μg/ml [Table 4]. A. actinomycetemcomitans was found to be more sensitive than P. gingivalis showing its susceptibility to the last dilution (1 μg/ml) set for the study. To determine the exact value of MIC for A. actinomycetemcomitans, further dilutions of <1 μg/ml are required.
Table 4.
MIC values of simvastatin against A. actinomycetemcomitans and P. gingivalis

Discussion
Severe periodontitis acts as an inflammatory focus in the oral cavity, that potentiates the atherosclerotic process by stimulation of humoral and cell-mediated inflammatory pathways.[18] The bacteria detected in atheromatic plaque lesions included many oral microbiota which principally comprised of anaerobic A. actinomycetemcomitans and P. gingivalis.[19] As per the regression model, a linear dose response gradient was observed between increasing numbers of sites with >20% of alveolar bone loss and coronary heart disease independent of other known risk factors.[20] This was further supported by National Health and Nutrition Examination Survey III, where a positive correlation was proved between periodontal disease and CVD.[21]
P. gingivalis secretes toxic products which contribute to inactivation of the effector molecules of the host immune response leading to tissue destruction. It can invade aortic and heart endothelial cells through fimbriae.[22] It also expresses a virulence factor called collagen like platelet aggregation associated protein, that induces platelet aggregation and plays role in atheroma formation.[23] Periodic P. gingivalis bacteremia induced aortic and coronary lesions consistent with atherosclerosis even in normocholesterolemic pigs.[24] This suggests their role in exerting atherogenic stimulus independent of high cholesterol levels. A. actinomycetemcomitans secretes leukotoxin, which was suggested to be in association between periodontitis and CVD.[25]
Limitations such as inaccessible areas, residual sub-gingival pathogens and inability to eliminate the bacteria that penetrated the host tissues, led to the initiation of antimicrobial therapy along with mechanical debridement. Widespread use of common antimicrobials independently for different infectious conditions disturbs the delicate ecologic equilibrium of the body, allowing the proliferation of resistant bacteria, development of super infections and systemic toxic effects.[26] Hence, it would be of great advantage to the patient, if a single drug could simultaneously take care of more than a single infection like periodontitis and other systemic disease.
Though presently age is not a primary determinant to classify periodontitis, most of the existing literature shows that periodontal disease affects the majority of the adult population above the age of 35-40 years.[27] 50% of the population older than 60 years are known to be the sufferers of periodontitis and of them, approximately 55% had either the diagnosis of atherosclerosis, or a history of stroke or acute coronary syndrome.[28] Although multiple causes have been recognized for initiation of CVD, the most common and modifiable risk factor associated is hyperlipidemia. In this modern era, due to the changing lifestyle, though hyperlipidemia is observed in the younger generations, it is predominantly detected in the age range of 50-70 years.[29] Hence, age range seems to be a common factor for the occurrence of both periodontitis and hyperlipidemia.
Statins are inhibitors of 3-hydroxy-3-methylglutarylcoenzymeA (HMG-CoA) reductase enzyme and prevents the formation of an intermediate product, mevalonate. This compound is also a precursor for other nonsteroidal isoprenoid compounds, farnesyl pyrophosphate and geranylgeranyl pyrophosphate which take part in protein prenylation, an important post translational modification, required for the biological functions of the cell.[30] Blocking of mevalonate formation inhibits protein prenylation that affects several signal transduction steps causing various pleiotropic effects such as improving endothelial function, immunomodulation, antioxidant activity, and treatment of malignancies.[10]
Simvastatin, a semi-synthetic statin on systemic administration improved bone mineral density in older women and reduced the risk of tooth loss in diabetes mellitus patients with chronic periodontitis.[31,32] It showed many beneficial effects to improve the health of periodontium through mechanisms like:
Periodontal regeneration; by augmenting bone morphogenetic proteins-Smad signaling that enhances bone formation, and antagonizing tumor necrosis factor-α (TNF-α) to Ras/Rho/mitogen activated protein kinase that causes osteoclastic differentiation. It also causes significant increase in levels of osteoblast differentiation factors such as alkaline phosphatase, osteopontin, osteocalcin and vascular endothelial growth factor.
Anti-inflammatory action; by reducing the proinflammatory cytokines like Interleukin-6 (IL-6) and IL-8 and down regulation of nuclear factor kappa β and activator protein 1 that are essential for IL-1α stimulated IL-6 and IL-8 expression.[33]
Minimum inhibitory concentration is important in diagnostic laboratories to confirm the resistance of microorganisms to an antimicrobial agent and also to monitor the activity of new antimicrobial agents.[6] MIC is a measure of the potency of an antimicrobial drug. Different bacterial species have varying MICs. Sensitive strains have relatively low MICs and resistant strains have relatively high MICs.
Most frequently employed antimicrobial drugs to treat periodontal disease are used at dosages ranging from 100 to 500 mg, twice or thrice a day [Table 1]. In contrast, the recommended usual dose of simvastatin is 10-80 mg once a day for its hypolipidemic activity.[34] In an in vitro study, a relatively low concentration of simvastatin (10−8 and 10−7M) promoted periodontal ligament cell proliferation and osteoblastic differentiation with increase in osteopontin, calcium, and alkaline phosphatase activity.[33] As a general rule of thumb, the concentration of antimicrobial drug in the blood should exceed the MIC by a factor of 2-8 times to offset the tissue barriers that restrict access to the infected site.[35] This higher concentration may sometimes lead to systemic toxic effects. In the present study, the MIC values of simvastatin obtained for P. gingivalis is higher and for A. actinomycetemcomitans, it is very much lower than the other antimicrobials [Table 4].
Clinically, periodontitis patients under statin therapy had a 37% less number of pathological periodontal pockets compared with those without under statin medication.[36] Improvement in the clinical parameters and infrabony defects fill was observed following scaling and root planing (SRP) with locally delivered simvastatin in periodontitis patients, compared with SRP plus placebo.[15] In a retrospective analysis with 7 years follow-up, systemic administration of simvastatin showed reduced risk of tooth loss in patients with chronic periodontitis.[37] Hence in this context, simvastatin may play a dual role in treating both hyperlipidemia to prevent CVD and periodontitis, which might prevent additional prescription of antimicrobials for periodontitis patients who were already under statin therapy. Statins were beneficial even in patients suffering from sepsis through immunomodulation by altering the function of both T cells and antigen-presenting cells.[38] They reduced the release of the proinflammatory cytokines such as TNF-α, IL-1, and IL-6 in rat models.[39]
It was found that bacterial HMG-CoA is 10,000 times weaker than the enzyme found in humans. Hence, it is unlikely to attribute hypolipidemic mechanism of action (i.e., inhibition of HMG CoA reductase) of statins to their antibacterial activity.[13] Previous studies have attributed the antimicrobial action of statins to increased bacterial clearance from the infected site[40] or by promoting the apoptosis of microbial cells.[12] In addition, hydrophobic nature of simvastatin also explained its antibacterial action, where it distresses the bacterial membrane in a “soap like” manner causing its death.[11] However, the exact mechanism of action needs further research.
The present in vitro MIC study helps us to focus on an intervention approach to design and conduct a clinical trial to detect the beneficial effect of simvastatin on patients at risk for periodontitis. However, in vitro values of MIC may not hold good for in vivo studies due to their inherent limitations. The growth of microorganisms in vitro is exponential, whereas the growth in vivo can be very slow to none.[41] Though MIC does not indicate the true activity of the drug at the locus of infection, the in vitro MIC serves as a surrogate marker attempting to quantify the drug activity.[42]
Scientifically based understanding of the etiopathogenesis of periodontal disease has laid a new responsibility on dentists to care for present and future periodontitis patients not only for their dental health, but also for the systemic health. Within limitations of the present study, lowest concentration of simvastatin was proven to be effective for both A. actinomycetemcomitans and P. gingivalis. However, since periodontitis is a polymicrobial disease, the susceptibility of various other periodontal pathogens to this drug must to be evaluated. Though the long-term safety profiles of statins are well-documented, further studies are required to: (a) Investigate the safety of using simvastatin in nonhyperlipidemic patients to treat periodontitis. (b) Assess the in vivo efficacy of simvastatin with other traditionally prescribed antimicrobials used for periodontal therapy. (c) Evaluate the in vivo effect of simvastatin in different formulations (gel, chips, strips, fibers, etc.,) with variable concentrations. The in vitro determination of their concentration in gingival crevicular fluid and serum samples might help us to know the ideal dosage and formulation required for antimicrobial and regenerative activity of simvastatin to treat periodontitis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Baehni P, Tsai CC, McArthur WP, Hammond BF, Taichman NS. Interaction of inflammatory cells and oral microorganisms. VIII. Detection of leukotoxic activity of a plaque-derived gram-negative microorganism. Infect Immun. 1979;24:233–43. doi: 10.1128/iai.24.1.233-243.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamont RJ, Oda D, Persson RE, Persson GR. Interaction of Porphyromonas gingivalis with gingival epithelial cells maintained in culture. Oral Microbiol Immunol. 1992;7:364–7. doi: 10.1111/j.1399-302x.1992.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 3.Rudney JD, Chen R, Sedgewick GJ. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect Immun. 2001;69:2700–7. doi: 10.1128/IAI.69.4.2700-2707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotic therapy in periodontics. Periodontol 2000. 1996;10:45–78. doi: 10.1111/j.1600-0757.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 5.Slots J, Rams TE. Antibiotics in periodontal therapy: Advantages and disadvantages. J Clin Periodontol. 1990;17:479–93. doi: 10.1111/j.1365-2710.1992.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 6.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl 1):5–16. doi: 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 7.Mombelli A. The use of antibiotics in periodontal therapy. In: Lindhe J, Karring T, Lang NP, editors. Clinical Periodontology and Implant Dentistry. UK: Munksgaard; 2003. pp. 106–49. [Google Scholar]
- 8.DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM. Dental disease and risk of coronary heart disease and mortality. BMJ. 1993;306:688–91. doi: 10.1136/bmj.306.6879.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–60. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 10.Grover HS, Luthra S, Maroo S, Maroo N. The pleotropic role of statins: Could it be the imminent host modulation agent in periodontics? Dent Res J (Isfahan) 2013;10:143–8. doi: 10.4103/1735-3327.113319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman P, Linde C, Pütsep K, Pohanka A, Normark S, Henriques-Normark B, et al. Studies on the antibacterial effects of statins – in vitro and in vivo. PLoS One. 2011;6:e24394. doi: 10.1371/journal.pone.0024394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masadeh M, Mhaidat N, Alzoubi K, Al-Azzam S, Alnasser Z. Antibacterial activity of statins: A comparative study of atorvastatin, simvastatin, and rosuvastatin. Ann Clin Microbiol Antimicrob. 2012;11:13. doi: 10.1186/1476-0711-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jerwood S, Cohen J. Unexpected antimicrobial effect of statins. J Antimicrob Chemother. 2008;61:362–4. doi: 10.1093/jac/dkm496. [DOI] [PubMed] [Google Scholar]
- 14.Shivanand S, Kumar AP, Jagadevappa P. Influence of method of preparation on solubility, physicochemical properties and in-vitro release profile of simvastatin-cyclodextrin inclusion complexes: A comparative study. Int J Chem Tech Res. 2010;2:562–71. [Google Scholar]
- 15.Pradeep AR, Thorat MS. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: A randomized clinical trial. J Periodontol. 2010;81:214–22. doi: 10.1902/jop.2009.090429. [DOI] [PubMed] [Google Scholar]
- 16.Song W, Condron S, Mocca BT, Veit SJ, Hill D, Abbas A, et al. Local and humoral immune responses against primary and repeat Neisseria gonorrhoeae genital tract infections of 17beta-estradiol-treated mice. Vaccine. 2008;26:5741–51. doi: 10.1016/j.vaccine.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorgensen JH, Ferraro MJ. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin Infect Dis. 2009;49:1749–55. doi: 10.1086/647952. [DOI] [PubMed] [Google Scholar]
- 18.Haynes WG, Stanford C. Periodontal disease and atherosclerosis: From dental to arterial plaque. Arterioscler Thromb Vasc Biol. 2003;23:1309–11. doi: 10.1161/01.ATV.0000087144.24654.71. [DOI] [PubMed] [Google Scholar]
- 19.Fiehn NE, Larsen T, Christiansen N, Holmstrup P, Schroeder TV. Identification of periodontal pathogens in atherosclerotic vessels. J Periodontol. 2005;76:731–6. doi: 10.1902/jop.2005.76.5.731. [DOI] [PubMed] [Google Scholar]
- 20.Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–37. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 21.Arbes SJ, Jr, Slade GD, Beck JD. Association between extent of periodontal attachment loss and self-reported history of heart attack: An analysis of NHANES III data. J Dent Res. 1999;78:1777–82. doi: 10.1177/00220345990780120301. [DOI] [PubMed] [Google Scholar]
- 22.Khlgatian M, Nassar H, Chou HH, Gibson FC, 3rd, Genco CA. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells. Infect Immun. 2002;70:257–67. doi: 10.1128/IAI.70.1.257-267.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herzberg MC, Meyer MW. Effects of oral flora on platelets: Possible consequences in cardiovascular disease. J Periodontol. 1996;67:1138–42. doi: 10.1902/jop.1996.67.10s.1138. [DOI] [PubMed] [Google Scholar]
- 24.Brodala N, Merricks EP, Bellinger DA, Damrongsri D, Offenbacher S, Beck J, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2005;25:1446–51. doi: 10.1161/01.ATV.0000167525.69400.9c. [DOI] [PubMed] [Google Scholar]
- 25.Johansson A, Kalfas S. Oral Health Care: Prosthodontics, Periodontology, Biology, Research and Systemic Conditions. Zagreb: InTech; 2012. Virulence mechanisms of leukotoxin from Aggregatibacter actinomycetemcomitans; pp. 165–92. [Google Scholar]
- 26.Barbosa TM, Levy SB. The impact of antibiotic use on resistance development and persistence. Drug Resist Updat. 2000;3:303–11. doi: 10.1054/drup.2000.0167. [DOI] [PubMed] [Google Scholar]
- 27.Heitz-Mayfield LJ, Schätzle M, Löe H, Bürgin W, Anerud A, Boysen H, et al. Clinical course of chronic periodontitis. II. Incidence, characteristics and time of occurrence of the initial periodontal lesion. J Clin Periodontol. 2003;30:902–8. doi: 10.1034/j.1600-051x.2003.00399.x. [DOI] [PubMed] [Google Scholar]
- 28.Ramesh A, Thomas B, Rao A. Evaluation of the association between chronic periodontitis and acute coronary syndrome: A case control study. J Indian Soc Periodontol. 2013;17:210–3. doi: 10.4103/0972-124X.113073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sreedevi K, Rao JV, Fareedullah MD, Vijayakumar S. A study on prescription pattern of statins in cardiovascular disease. Der Pharm Lettre. 2011;3:393–6. [Google Scholar]
- 30.Stancu C, Sima A. Statins: Mechanism of action and effects. J Cell Mol Med. 2001;5:378–87. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan KA, Andrade SE, Boles M, Buist DS, Chase GA, Donahue JG, et al. Inhibitors of hydroxymethylglutaryl-coenzyme A reductase and risk of fracture among older women. Lancet. 2000;355:2185–8. doi: 10.1016/S0140-6736(00)02400-4. [DOI] [PubMed] [Google Scholar]
- 32.Chung YS, Lee MD, Lee SK, Kim HM, Fitzpatrick LA. HMG-CoA reductase inhibitors increase BMD in type 2 diabetes mellitus patients. J Clin Endocrinol Metab. 2000;85:1137–42. doi: 10.1210/jcem.85.3.6476. [DOI] [PubMed] [Google Scholar]
- 33.Elavarasu S, Suthanthiran TK, Naveen D. Statins: A new era in local drug delivery. J Pharm Bioallied Sci. 2012;4:S248–51. doi: 10.4103/0975-7406.100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao SK, Prasad T, Mohanta GP, Manna PK. An overview of statins as hypolipidemic drugs. Int J Pharm Sci Drug Res. 2011;3:178–83. [Google Scholar]
- 35.Neu HC. Current practices in antimicrobial dosing. Rev Infect Dis. 1981;3:12–8. doi: 10.1093/clinids/3.1.12. [DOI] [PubMed] [Google Scholar]
- 36.Lindy O, Suomalainen K, Mäkelä M, Lindy S. Statin use is associated with fewer periodontal lesions: A retrospective study. BMC Oral Health. 2008;8:16. doi: 10.1186/1472-6831-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunha-Cruz J, Saver B, Maupome G, Hujoel PP. Statin use and tooth loss in chronic periodontitis patients. J Periodontol. 2006;77:1061–6. doi: 10.1902/jop.2006.050280. [DOI] [PubMed] [Google Scholar]
- 38.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: From protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6:358–70. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medeiros BV, Azevedo IM, Rego AC, Filho IA, Ferreira MC, Medeiros CA. Attenuation of lung injury using simvastatin in a rat sepsis model. J Surg Clin Res. 2012;3:1–8. [Google Scholar]
- 40.Chaudhry MZ, Wang JH, Blankson S, Redmond HP. Statin (cerivastatin) protects mice against sepsis-related death via reduced proinflammatory cytokines and enhanced bacterial clearance. Surg Infect (Larchmt) 2008;9:183–94. doi: 10.1089/sur.2006.077. [DOI] [PubMed] [Google Scholar]
- 41.Levison ME. Pharmacodynamics of antimicrobial drugs. Infect Dis Clin North Am. 2004;18:451–65. doi: 10.1016/j.idc.2004.04.012. vii. [DOI] [PubMed] [Google Scholar]
- 42.Briethaupt H. The new antibiotics: Can nivel antibacterial treatment combat the rising tide of drug resistant infections? Nat Biotechnol. 1999;17:1165–9. doi: 10.1038/70705. [DOI] [PubMed] [Google Scholar]


